During the course of constructing a device intended to generate heat resistively, I noticed a tiny voltage present in the device between the ground plane and both ends of the resistive element.
The element itself is made from pure carbon, bonded and stabilised with a SiO2 cement. It is designed to operate up to 350c, so I chose a flue cement that is rated to 1500c over polymerised silicon that is rated to 300c or so. The element is cast on a bed of pure SiO2 cement that insulates it electrically from the brass tube the element is intended to heat. It also has a small glass-encapsulated thermistor bead embedded in it, which turned out to be completely superfluous...
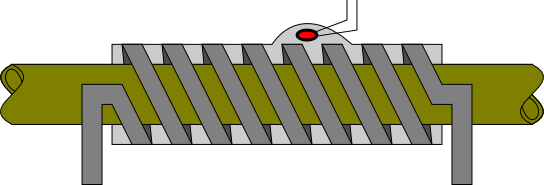
Basically what I did was take a piece of brass tube and wind two pieces of thick square flexible plastic around it. I then removed one of them after taping the ends down, leaving an even spiral groove which I filled with the cement and left to cure. Then I removed the remaining bit of plastic and filled the groove left behind with a mixture of a little flue cement, graphite and thinners. I added wires, and when it had cured I stuck the thermistor and wires on with more cement and left it to go off completely. I was pretty pleased with the results by now...
I had already made several test castings on plastic and paper formers and established this format of element to be around 15KOhm resistance, but here I couldnt get a clear reading and the meter jumped around like it was connected to a live component. I tested for resistance between the element and tube, expecting a short and got even jumpier readings, so I tested the tube for voltage and found a tiny milliamp current at 0.2V. Eventually I traced it, after disconnecting everything, to a differential between the tube and element. I assumed it to be electrolytic and coming from traces of moisture in the fresh cement and ignored it, but it never went away.
Not electrolytic then. The voltage also increased with the tube at a higher temperature, so I thought it was behaving like a thermocouple. It isnt a thermocouple in a classic sense though, it would be a semiconductor analogue of one. It is also extremely inefficient in this format, and probably only works as such because of an accident.
I used a piece of marine brass modelling stock for the tube, figuring that the Monel it is made of would resist better chemically inside. Monel has the addition of around 15% Aluminium compared with the little or none in ISO brass formulations, and forms a layer of Aluminium Oxide (Carborundum, or Sapphire) a few molecules thick on the surface of the Monel that completely passivates it. It was this that made the difference.
I made some test pieces with clean and oxidised metals and measured them, and discovered that Lead (passivated and clean results being identical) is by far the best substrate, with Aluminium coming in a close second. I just pasted the unmodified cement onto the test substrate, left it to cure and covered it with the carbon:
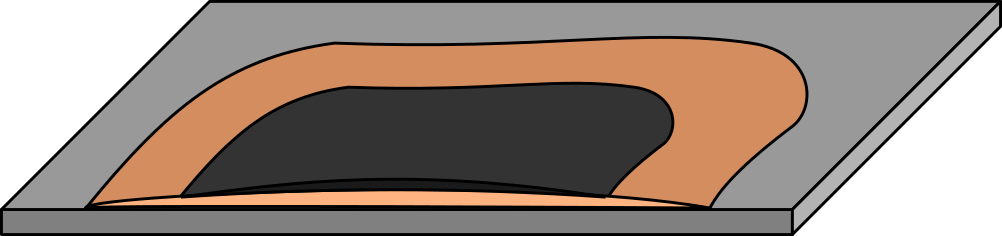
Strangely, Copper, ISO Brass, Tin, Zinc and Stainless Steel were in the centivolt range, where Lead and Aluminium both were around half a volt. This almost directly contradicts results I have had experimenting with Aluminium, Stainless Steel, Magnesium, Copper and Carbon electrolytically, and makes little sense with the range of materials used in conventional thermocouples too.
Stainless Steel and Aluminium are opposite each other on the Voltaic chart, however the polarity is reversed, and Aluminium makes such a pitiful thermocouple it isnt commercially used – Copper, Iron and Nickel are more normal choices. It was this that clued me in to what may be happening inside the junction, along with the knowledge that it required an oxide layer to work. This is an insulator normally, so something odd was going on. Lead Oxide is also not known for its conductive qualities outside of Memristor technologies (I tested for Memristor activity and found none, however the configuration may be preventing this. Sulphur is also implicated in the chemical geometry that forms crystals known to 'memrist' along with Copper, Lead and Zinc.)
I wondered if perhaps it was a semiconductor thermocouple, but it didnt seem likely even though it reacted to heat with an increase in voltage. Microscopic examination of the cement revealed it to be finely crushed Silica, and was all sharp edges. This meant that despite the large surface area of the test pieces compared to the alloy bead of a normal thermocouple, there is very little contact between it and the metal surface. Even if there was conventional coupling, it could never do so at the levels I was seeing even at hundreds of degrees C.
Research had drawn a blank by now, leaving me to ponder on the ancient
pre-semiconductor research for a solution. I knew that old radio
circuits that used crystals relied on a point contact – the cat
whisker – to work. It is a primitive version of a Tunnel Diode, a
device that uses material geometry to achieve its purpose.
I think this is where the Carbon comes in. It is much finer particles than the Silicon, more
conductive and is packed against one side of the crystals only. This
enables it to make a decent contact and directly transfer electron
charge in bulk. Where it does make contact with the substrate, it
cannot transfer electron charge because it doesnt have the right
geometry to tunnel.
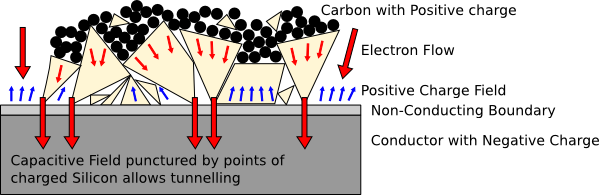 When an electron is pushed outside of a point boundary it is inside a
capacitive field that repels it into the lattice of the insulator.
The crystal geometry and thickness of this dielectric boundary
determine whether the electron can be grabbed and pulled into the
lattice of the conductor underneath. Any other electrons are repelled
away from the capacitive field that exists across a charged
dielectric, so that flat surface is non-conductive.
When an electron is pushed outside of a point boundary it is inside a
capacitive field that repels it into the lattice of the insulator.
The crystal geometry and thickness of this dielectric boundary
determine whether the electron can be grabbed and pulled into the
lattice of the conductor underneath. Any other electrons are repelled
away from the capacitive field that exists across a charged
dielectric, so that flat surface is non-conductive.
The co-incidence of both Lead and Aluminium oxides being used to provide capacitive boundaries elsewhere in the electronics industry is probably significant...
The Lead test piece is now three years old and still producing a voltage. I'd be interested if anyone else could replicate these experiments and shed some light on what I'm seeing.
 Morning.Star
Morning.Star
Discussions
Become a Hackaday.io Member
Create an account to leave a comment. Already have an account? Log In.