Somewhere in Portland, there's a restaurant that serves grasshopper sushi rolls. Is it safe? Is it good? I don’t know!
Because I am a grasshopper researcher this summer in Ann Arbor, Michigan, I have other questions in mind: How do people catch these bugs? If you’ve ever tried to catch one, you know that it is nearly uncatchable when its skeletal muscles get to work as you approach with silent steps, trying to capture it for an afternoon snack.
Catching grasshoppers in Ann Arbor is my exciting challenge this summer. Finding out why they are hard to catch is my neuroscience project.
Background:
Why are they hard to catch? Because they can quickly jump away when a person or another insect or object approaches it. How are they able to quickly hop away to escape a potential predator or avoid collision with an object? To address this specific question, I will look into the movement detector neurons in the grasshopper’s brain—the organ that fascinates me.
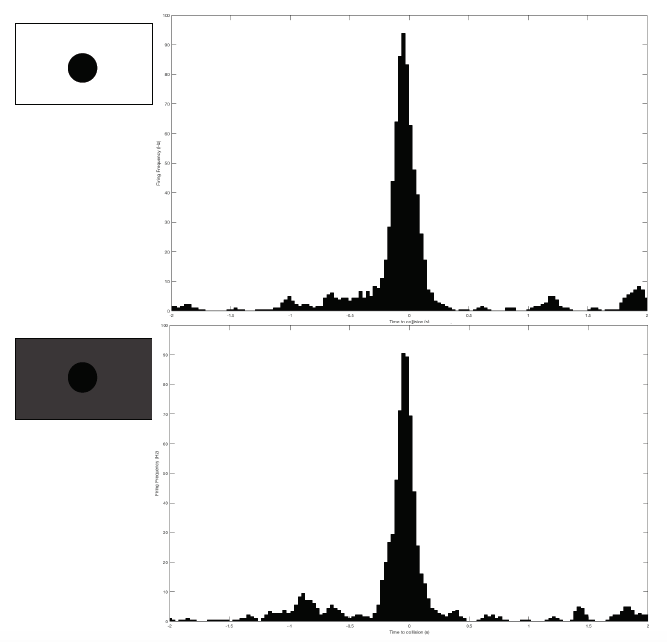
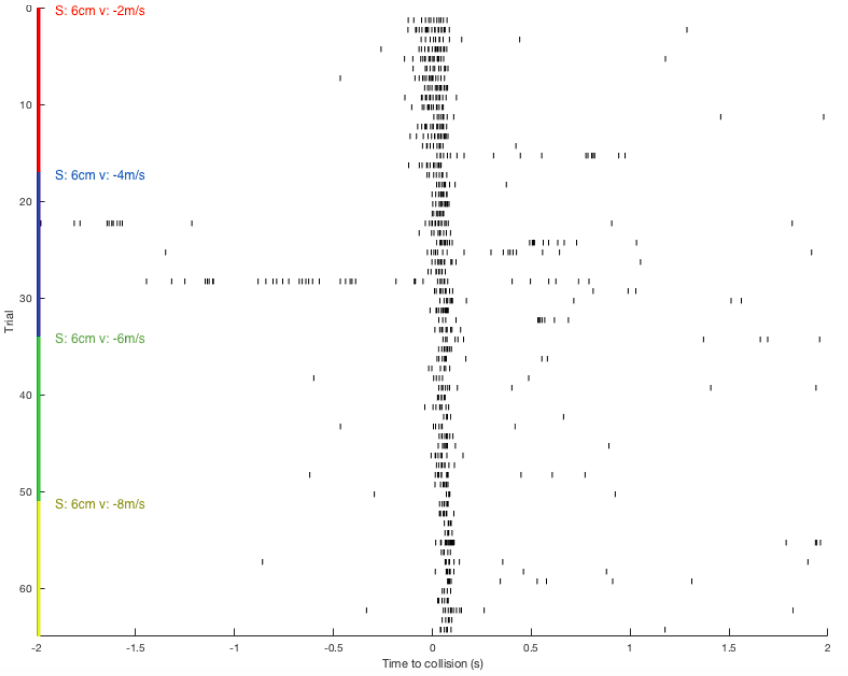
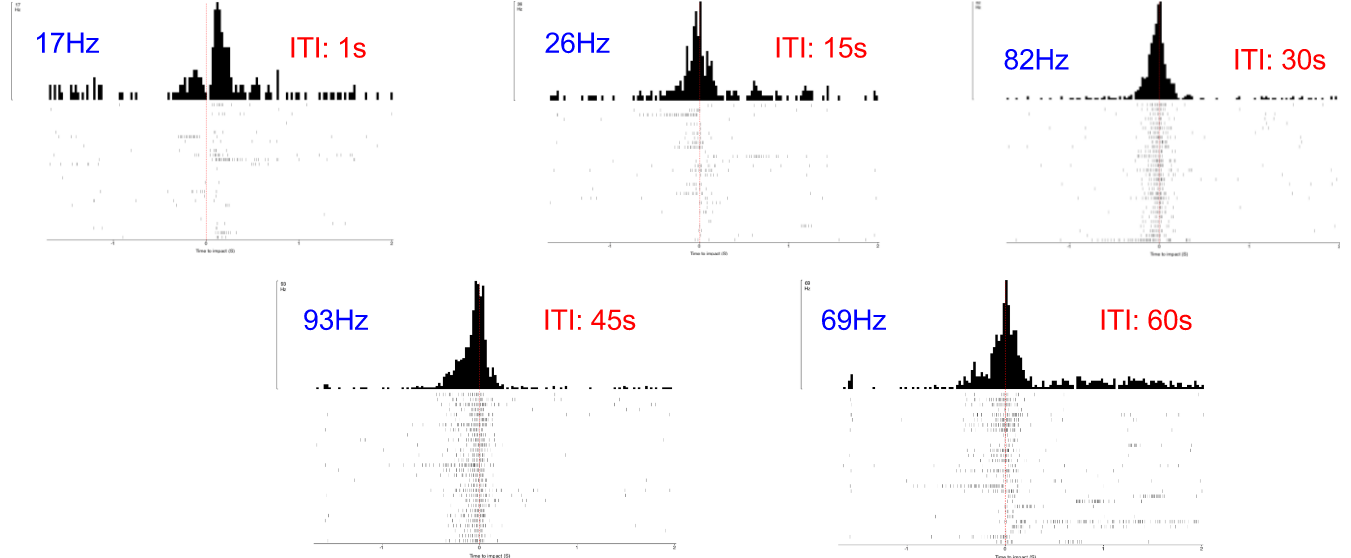
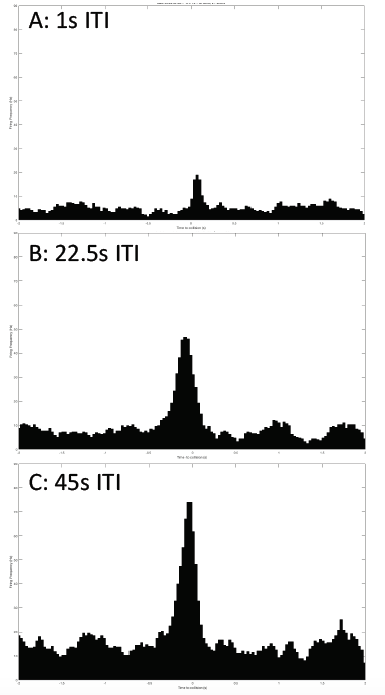
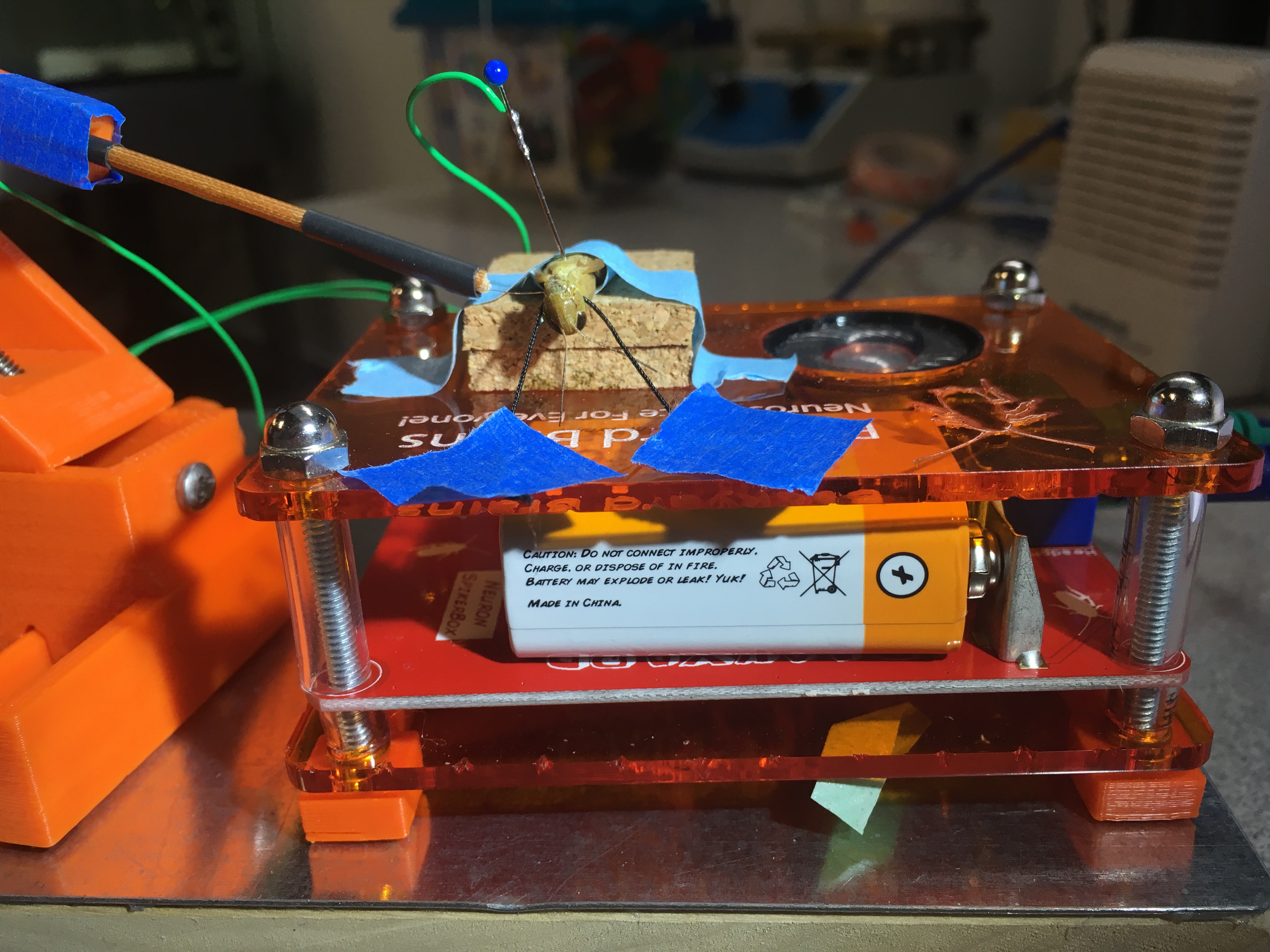
Just as I can see it with my eyes, the grasshopper can see me if I come to it. Or if I show it scenes from Star Wars when spaceships are flying toward the viewer, the grasshopper can see them too and would hop, hop away. That is what researchers Rind and Simmons found in 1992 in their research on the vision of the locust, or a kind of grasshoppers that form swarms. The grasshopper’s nervous system includes a type of visual neurons, called descending contralateral movement detector (DCMD), that receives visual info from the eyes and sends that info to the legs, and underlies the grasshoppers’ ability to visually detect and react to an approaching object, be it a spider looking for a crunch or an astronomically speedy spaceship.
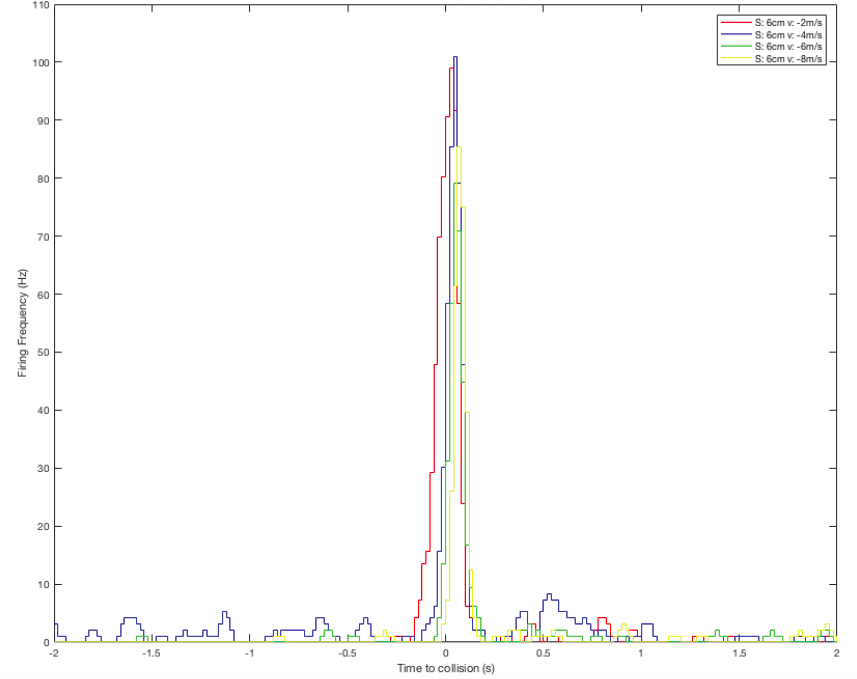
In human language, the brains of these bugs are capable of serious mathematics. In a paper published in 1995, researcher Hatsopoulos and colleagues came up with an equation that describe how the DCMD neurons sense and respond to approaching and receding objects: velocity, or speed, of the approaching image:
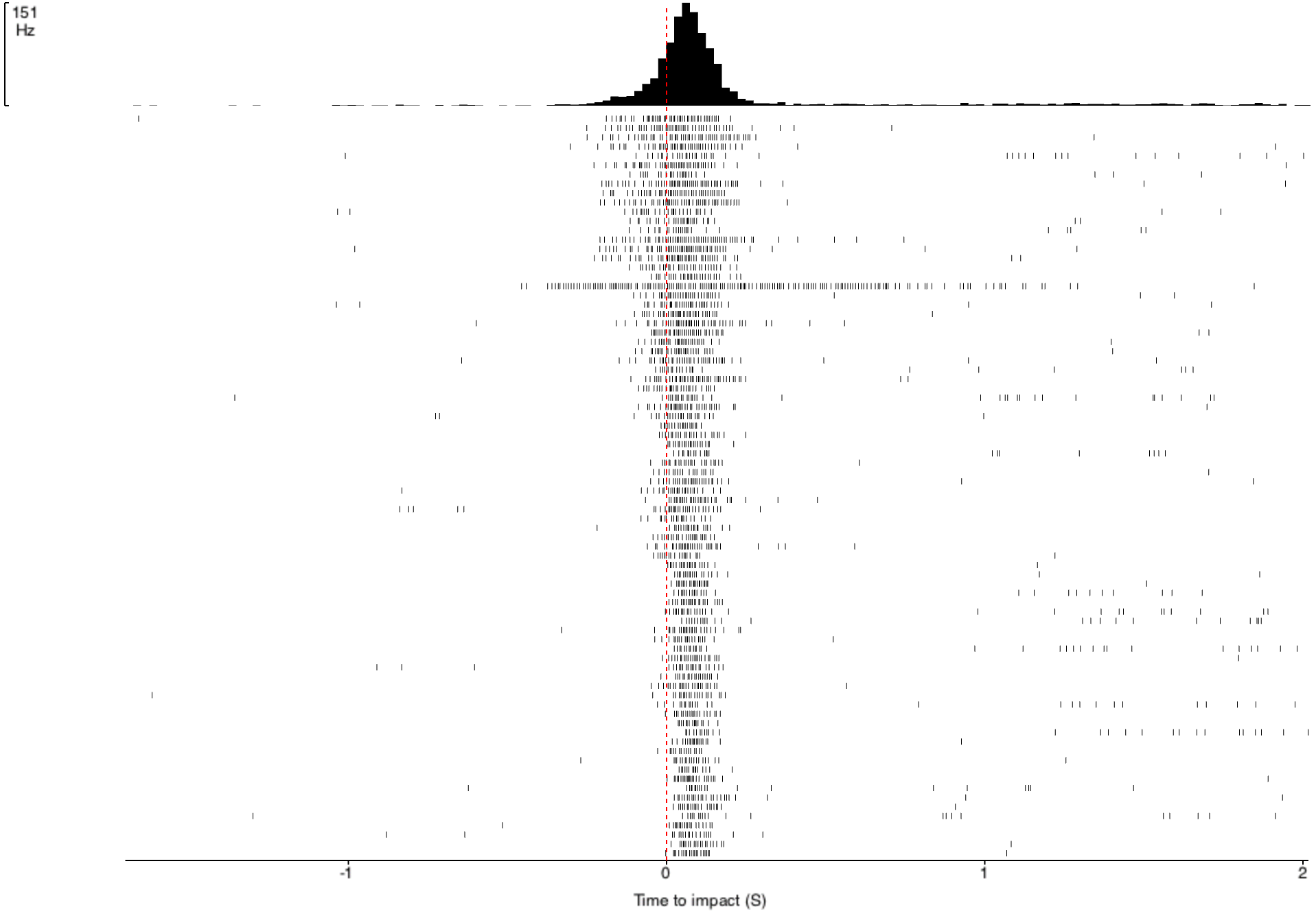
multiplied by an exponential function of size of object’s image on the retina: On a high-level consideration of the computational way the brain of the grasshopper functions, the activity of the DCMD neuron is related to how fast the image is coming toward the eye of the grasshopper and the image size on the eye that changes with decreasing distance between object and the eye. The peak in firing is reached before the collision of the object and the grasshopper, and the bug can leap away using their legs to avoid being hit or eaten.
My goals:
In the world of scientific research, disagreements founded upon experimental evidence and thoughtful arguments give rise to scientific progress. In the two above-cited papers, I see several discrepancies between the two groups of researchers. While Rind and Simmons concluded that there was good correlation between the neuron’s activity and the object's acceleration during the exposure of the grasshopper to approaching objects, Hatsopoulos and colleagues used both their computation and experiment to conclude that the correlation was poor. The two papers generally agree that the DCMD neuron’s responses depend on the size and speed of the object. Keeping these ideas in mind, I will see what results my project will yield and I look forward to contributing to the discussion.
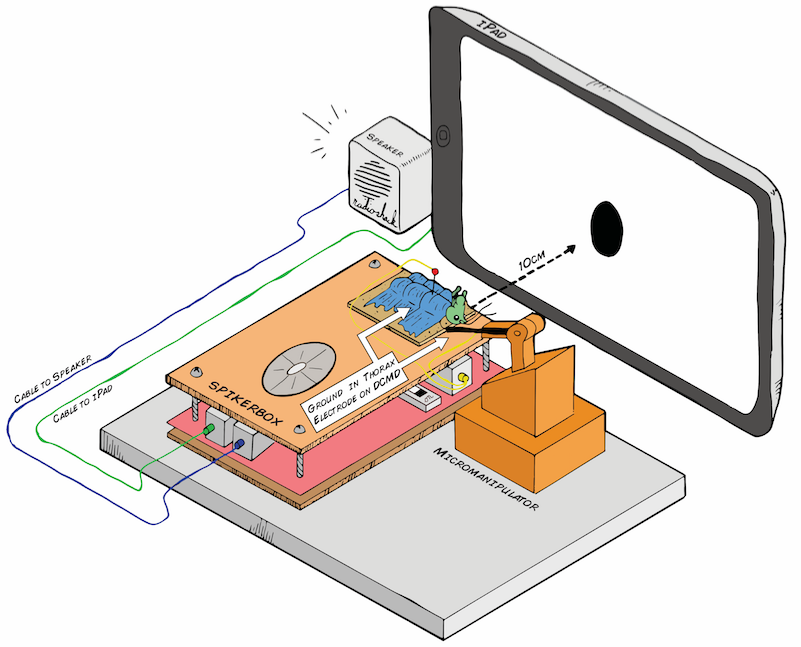
Art by Tanner @ All Hands Active, Ann Arbor, MI
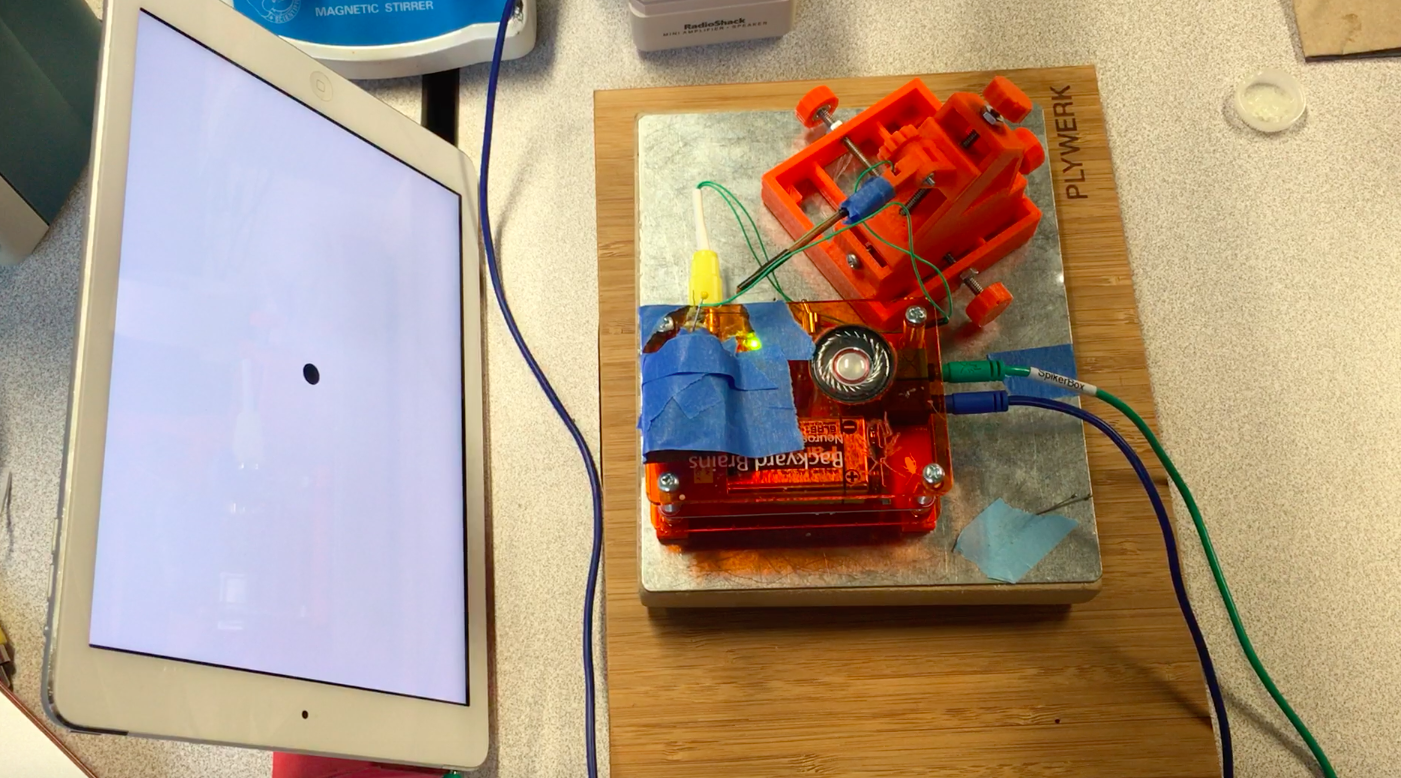
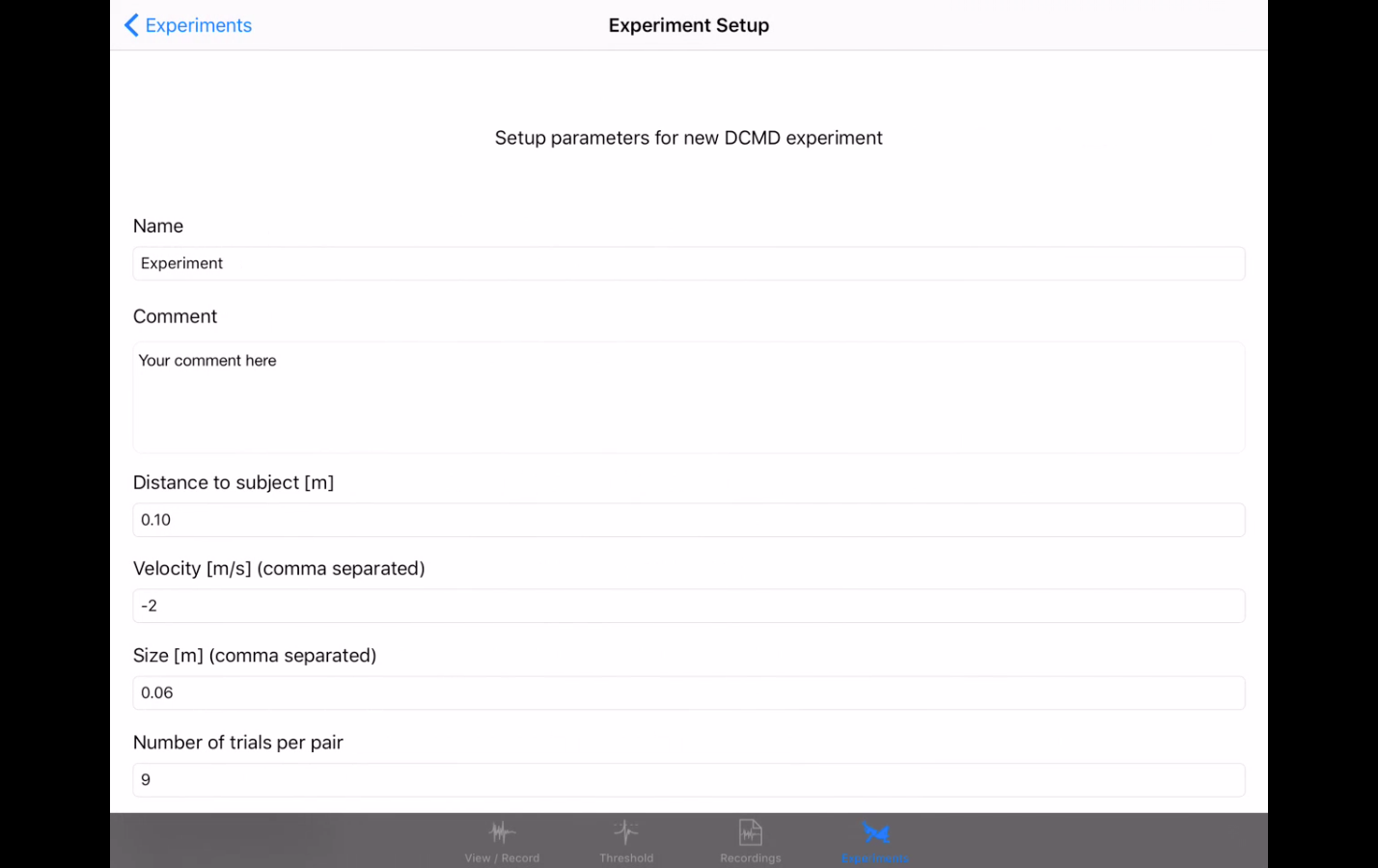
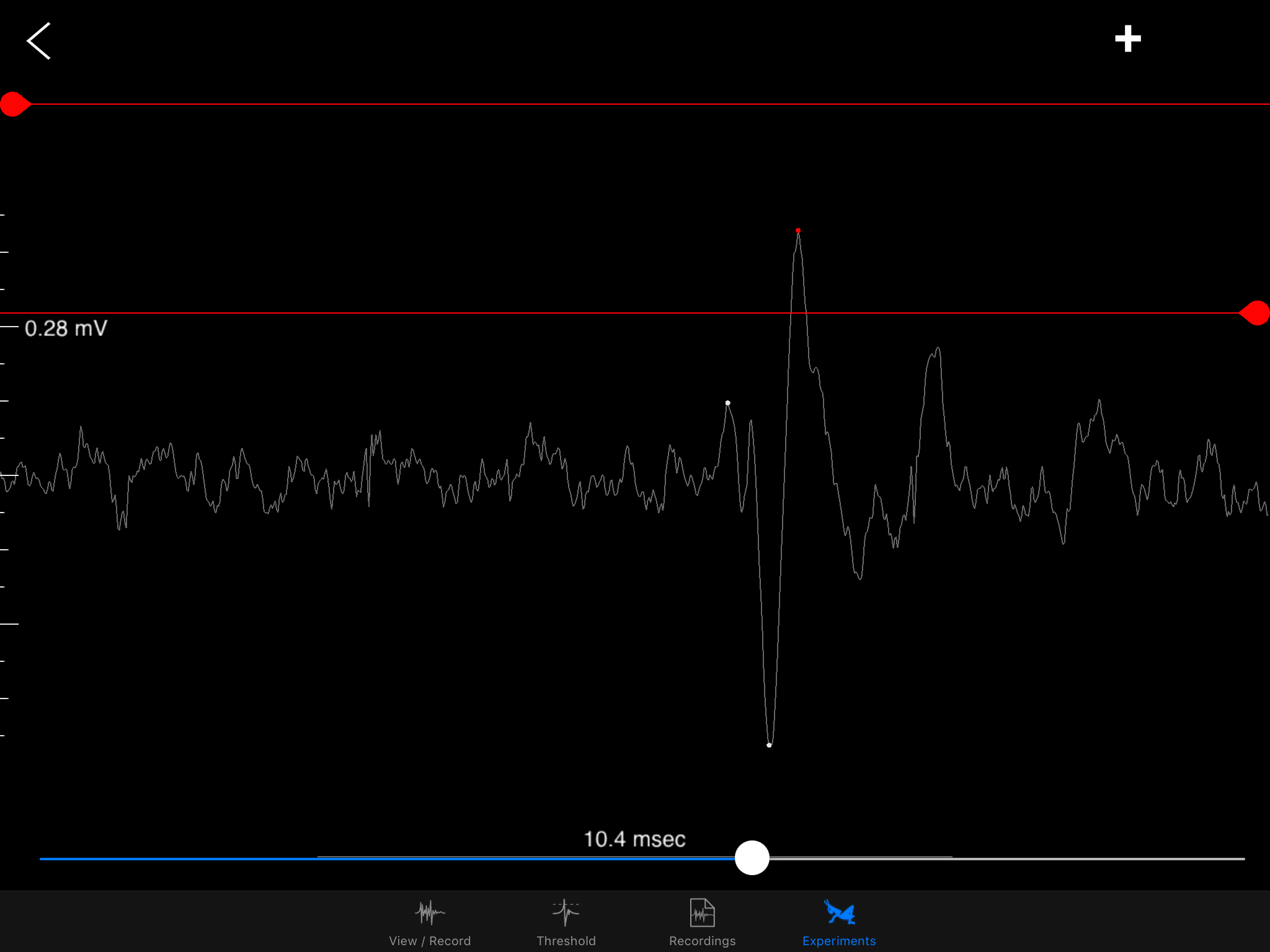
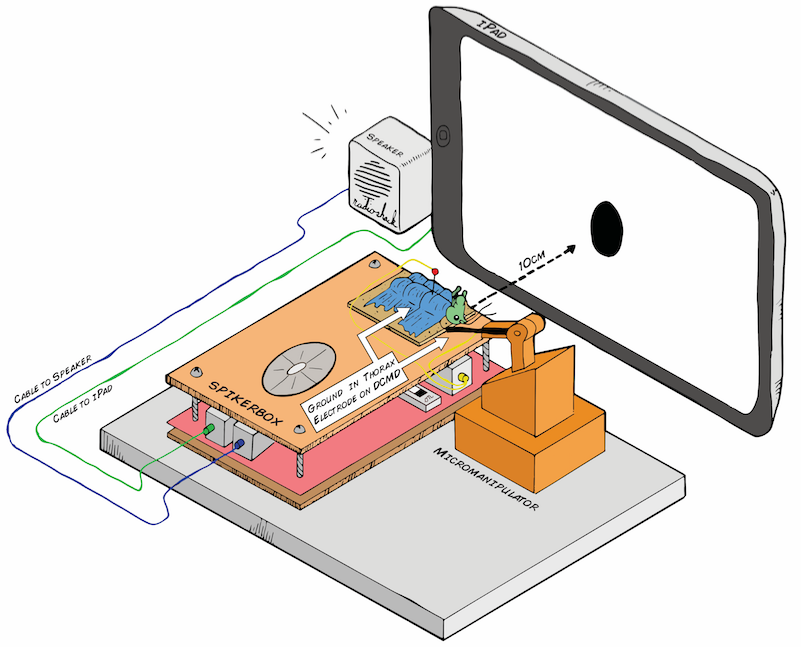
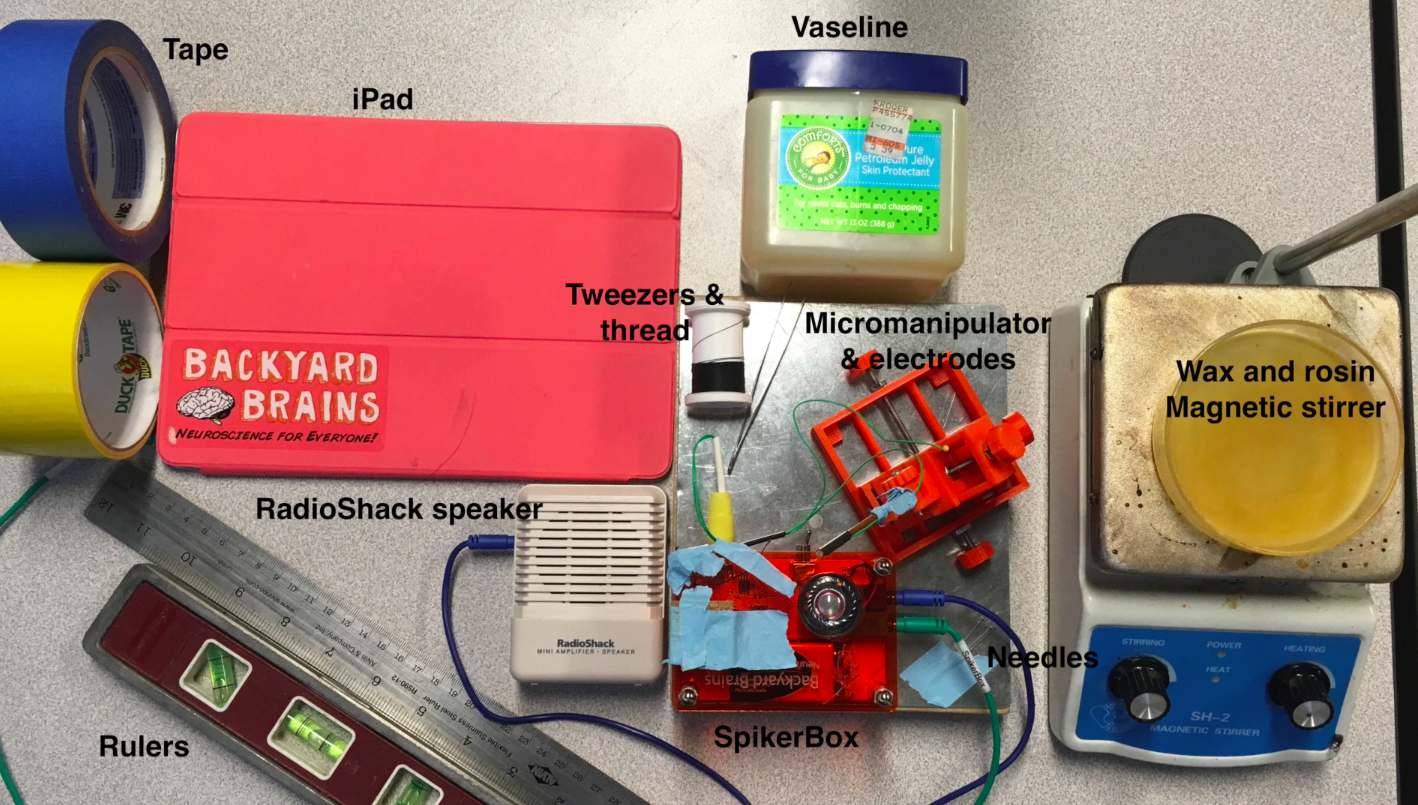
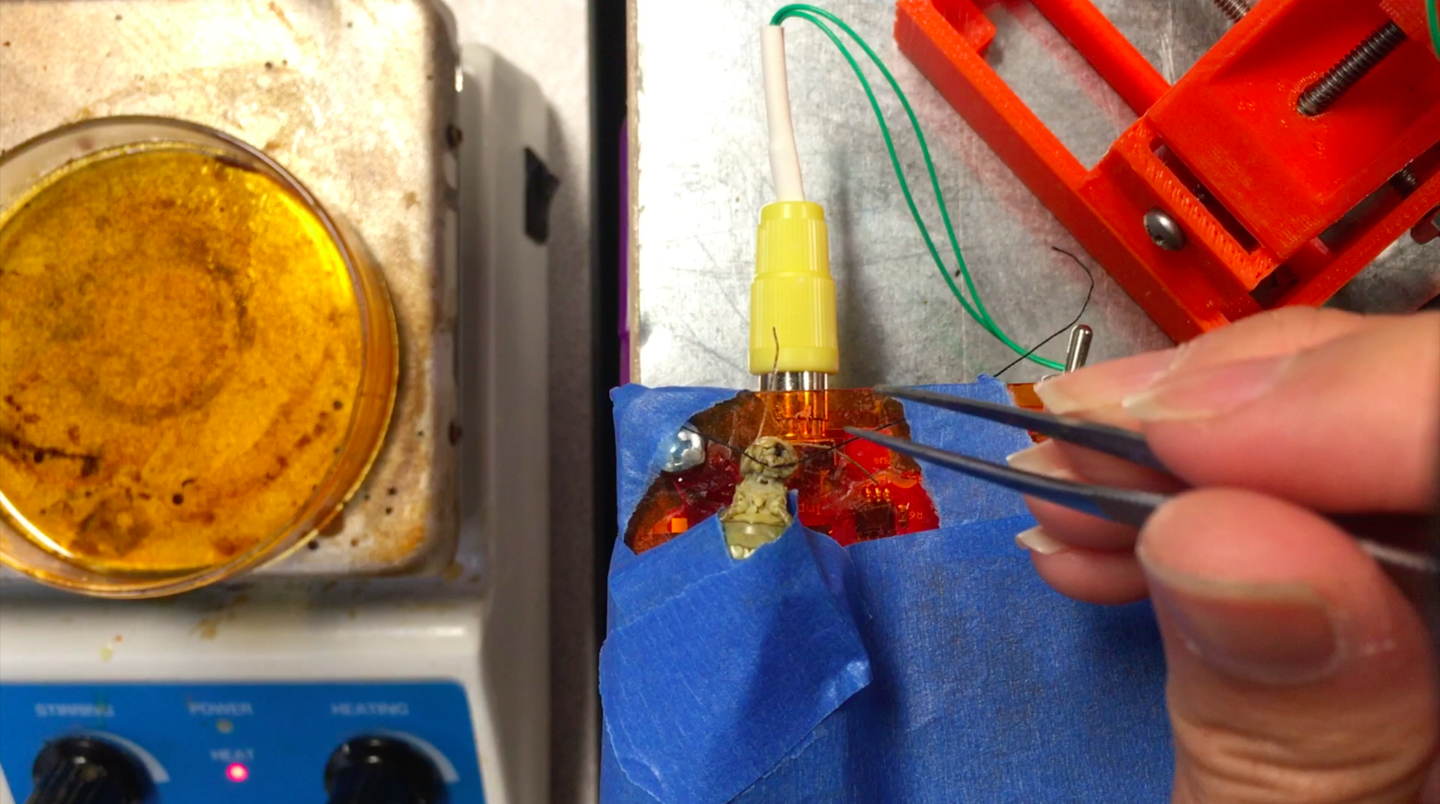
I hope to demonstrate that a fun and educational neuroscience project can be done outside of the far far away university labs! I use Backyard Brains’ Neuron SpikerBox that amplifies and visualizes the activity of the DCMDs in the form of electrochemical action potentials, or spikes. I also have an iPad with the SpikeRecorder app, which provides visual stimuli (growing or receding black dots on a white background) in front of the grasshopper's eye as well as records the DCMD activity.
Hop, hop away. This is how the grasshopper stays alive. This is how it continues to exist and eats plants and destroys our crops. But it...
Read more » Dieu My Nguyen
Dieu My Nguyen


 Sorting a bunch of spikes at once:
Sorting a bunch of spikes at once: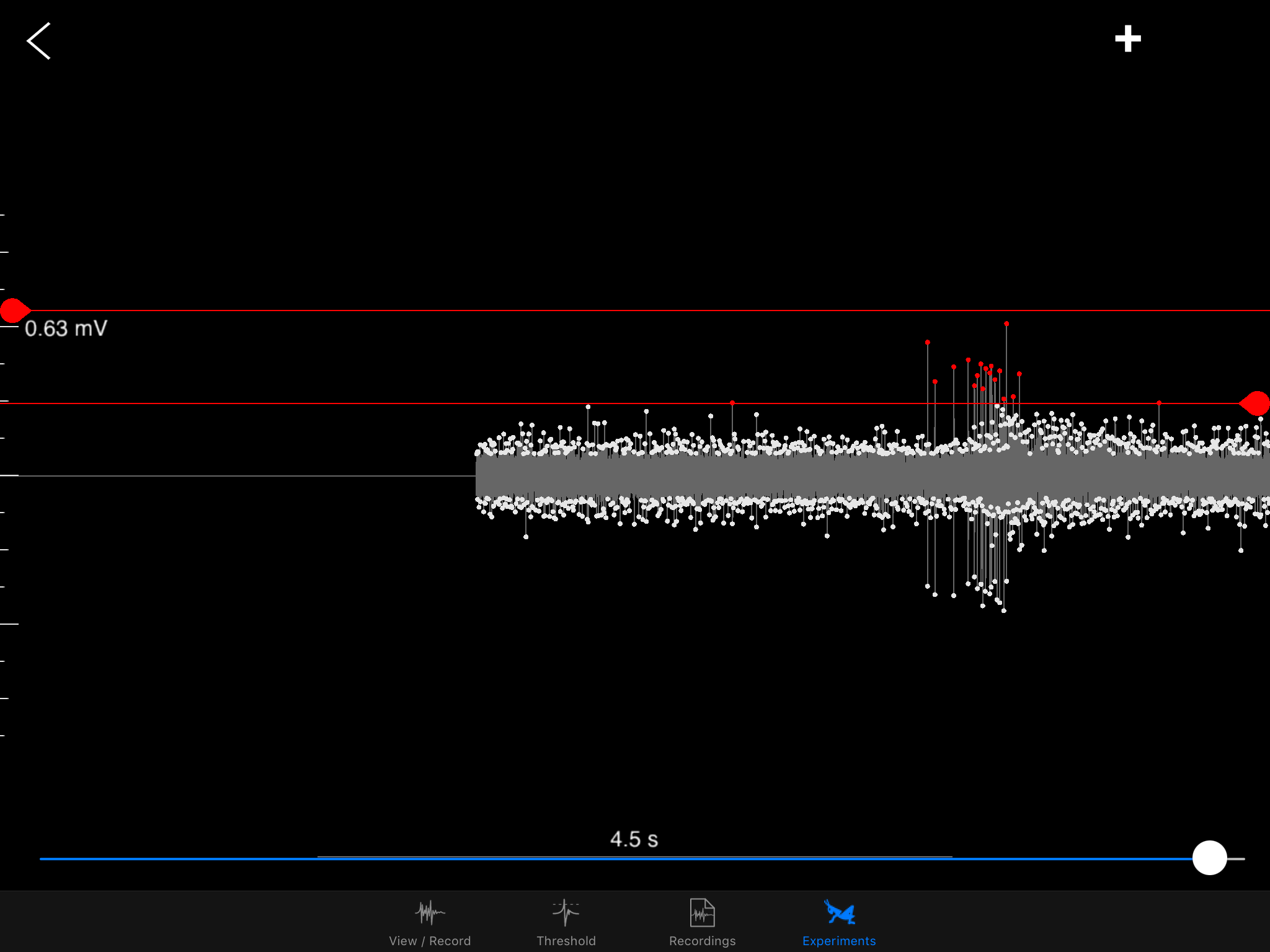











 Ashwin K Whitchurch
Ashwin K Whitchurch
 artbyphysicistkitty
artbyphysicistkitty
 johnowhitaker
johnowhitaker
 Amanda Brief
Amanda Brief
I am sorry my comment is not going to be about neuroscience or anything related to technology, nonetheless i like your project. The point is, i feel sad for the grasshopers ...