 Mods please delete my last link to licap, it's is incorrect and wrong page i linked. these are standard ultracaps.
Mods please delete my last link to licap, it's is incorrect and wrong page i linked. these are standard ultracaps.
 @ILove Scotch that lithium ion capacitor is not a battery, nor a hybrid. It's a capacitor. Look at that pathetic energy density. You're just shuttling ions, not doing reactions
@ILove Scotch that lithium ion capacitor is not a battery, nor a hybrid. It's a capacitor. Look at that pathetic energy density. You're just shuttling ions, not doing reactions
 So can you share a little more about how you got mixed up in electrochemistry? Sounds like it might be a cautionary tale...
So can you share a little more about how you got mixed up in electrochemistry? Sounds like it might be a cautionary tale...
 tbh, my first exposure to it was when I was a little kid and my dad showed me the penny/salt water on a bit of paper towel/nickel battery would make a voltage
tbh, my first exposure to it was when I was a little kid and my dad showed me the penny/salt water on a bit of paper towel/nickel battery would make a voltage
 within a year I decided, hey how about a sheet of aluminum, a sheet of copper, and bleach? Got enough to run a model motor but fast!
within a year I decided, hey how about a sheet of aluminum, a sheet of copper, and bleach? Got enough to run a model motor but fast!
 My understanding is that all batteries -- going back 200 years depend on electrochemistry. But if the pundits are to be believed, there's a movement towards quantum (solid-state) batteries that do not make use of ionic movement or chemical reactions. Do you know anything about these next-generation of energy storage devices?
My understanding is that all batteries -- going back 200 years depend on electrochemistry. But if the pundits are to be believed, there's a movement towards quantum (solid-state) batteries that do not make use of ionic movement or chemical reactions. Do you know anything about these next-generation of energy storage devices?
 no I don't. You have linky?
no I don't. You have linky?
 Also -- if someone wanted to study battery technology -- what should they start reading to better understand the lingo & problems battery engineers try to address?
Also -- if someone wanted to study battery technology -- what should they start reading to better understand the lingo & problems battery engineers try to address?
 so, let's get a few things straight- a solid electrolyte does not a solid state battery make. There are volume changes on *all* battery electrodes depending on the state of charge
so, let's get a few things straight- a solid electrolyte does not a solid state battery make. There are volume changes on *all* battery electrodes depending on the state of charge
 not heard of that before sounds interesting, just looking at - https://physicsworld.com/a/quantum-batteries-harvest-energy-from-light/
not heard of that before sounds interesting, just looking at - https://physicsworld.com/a/quantum-batteries-harvest-energy-from-light/
 Well -- one example: https://patentimages.storage.googleapis.com/22/0c/1d/f485c30d1ed67c/US10395850.pdf
Well -- one example: https://patentimages.storage.googleapis.com/22/0c/1d/f485c30d1ed67c/US10395850.pdf
 also, the definition of an anode, you know, the one Michael Faraday gave it, is an electrode that does an oxidation. An electrode that does a reduction is a cathode.
also, the definition of an anode, you know, the one Michael Faraday gave it, is an electrode that does an oxidation. An electrode that does a reduction is a cathode.
 (Sorry don't have anything immediately on hand that isn't marketing spiel from some place, so I went to academia)
(Sorry don't have anything immediately on hand that isn't marketing spiel from some place, so I went to academia)
 it doesn't matter what the voltage of the electrodes are, or if they're charging or discharging, all that matters is what they're doing- oxidation or reduction
it doesn't matter what the voltage of the electrodes are, or if they're charging or discharging, all that matters is what they're doing- oxidation or reduction
 so despite the fact that the material scientists and others don't know their terminology...
so despite the fact that the material scientists and others don't know their terminology...
 Dave I meant this earlier https://en.wikipedia.org/wiki/Lithium-ion_capacitor "The negative electrode or anode of the LIC is the battery type or high energy density electrode. The anode can be charged to contain large amounts of energy by reversible intercalation of lithium ions. This process is an electrochemical reaction."
Dave I meant this earlier https://en.wikipedia.org/wiki/Lithium-ion_capacitor "The negative electrode or anode of the LIC is the battery type or high energy density electrode. The anode can be charged to contain large amounts of energy by reversible intercalation of lithium ions. This process is an electrochemical reaction."
 this more informal and ever expanding definition of "battery" and other terms kind of muddles the water. I know I can count on you all to fight the good fight!
this more informal and ever expanding definition of "battery" and other terms kind of muddles the water. I know I can count on you all to fight the good fight!
 hey @ILove Scotch OK, but still...they're stretching the definition there. Intercalation takes time. I'd like to see how fast they can charge or discharge it. If it's a battery, it's got a hard upper limit, otherwise you'll plate lithium out and screw up the cell
hey @ILove Scotch OK, but still...they're stretching the definition there. Intercalation takes time. I'd like to see how fast they can charge or discharge it. If it's a battery, it's got a hard upper limit, otherwise you'll plate lithium out and screw up the cell
 double layer charging, even in solution, is much faster than a chemical reaction
double layer charging, even in solution, is much faster than a chemical reaction
 it has properties of both, hence hybrid.
it has properties of both, hence hybrid.
 I would hope it cycles faster and longer than a Li battery
I would hope it cycles faster and longer than a Li battery
![]() "New High-Performance Solid-State Battery Surprises the Engineers Who Created It" with link at https://scitechdaily.com/new-high-performance-solid-state-battery-surprises-the-engineers-who-created-it/
"New High-Performance Solid-State Battery Surprises the Engineers Who Created It" with link at https://scitechdaily.com/new-high-performance-solid-state-battery-surprises-the-engineers-who-created-it/
 I mean, at the moment Li ion batteries have Li ions intercalate into graphite on the negative side, and metal oxides of phosphates on the positive side
I mean, at the moment Li ion batteries have Li ions intercalate into graphite on the negative side, and metal oxides of phosphates on the positive side
 conversion type electrodes (FeF3), sulfur or Li air? those things give you a different product upon discharge
conversion type electrodes (FeF3), sulfur or Li air? those things give you a different product upon discharge
 Fe and LiF, Li2Sx, and Li2O2, respectively
Fe and LiF, Li2Sx, and Li2O2, respectively
 What chemistries are realistic to expect in 5-10 years for C&I storage?
What chemistries are realistic to expect in 5-10 years for C&I storage?
 what does C&I mean
what does C&I mean
 Commercial and Industrial.
Commercial and Industrial.
 ah
ah
 I assume that batteries will continue to be made of multiple small units, serialized and parallelized into large power/energy blocks.
I assume that batteries will continue to be made of multiple small units, serialized and parallelized into large power/energy blocks.
 it's interesting, Li ion has become so damn cheap and long life, it's a tough nut for the other stationary storage types to chase
it's interesting, Li ion has become so damn cheap and long life, it's a tough nut for the other stationary storage types to chase
![]() what about molten metal batteries? that guy from MIT came up with a simple design with liquid electrodes, density-separated, and liquid molten-salt electrolyte. no membranes, no electrode lattice degradation, no expensive materials, suitable for grid storage.
what about molten metal batteries? that guy from MIT came up with a simple design with liquid electrodes, density-separated, and liquid molten-salt electrolyte. no membranes, no electrode lattice degradation, no expensive materials, suitable for grid storage.
 credit where credit is due- Tesla has done some amazing stuff with 18650s. They still use those in the model S
credit where credit is due- Tesla has done some amazing stuff with 18650s. They still use those in the model S
 @Thomas Shaddack are those a flow battery?
@Thomas Shaddack are those a flow battery?
 folks have always wanted to do sulfur batteries with molten sulfur. Sodium-sulfur with both of them molten
folks have always wanted to do sulfur batteries with molten sulfur. Sodium-sulfur with both of them molten
 Sodium melts at 97C. Convenient
Sodium melts at 97C. Convenient
![]() @Dave Sopchak Nope. It's the Ambri thing.
@Dave Sopchak Nope. It's the Ambri thing.
 The ideas of all the connectors on the 18650 blocks give me the heebe-jeebies. I don't have real data for my concerns though.
The ideas of all the connectors on the 18650 blocks give me the heebe-jeebies. I don't have real data for my concerns though.
 "Undecided with Matt Ferrell" oh god, he gets so many things wrongs at times...
"Undecided with Matt Ferrell" oh god, he gets so many things wrongs at times...
 I've heard of Ambri, but don't know specifics
I've heard of Ambri, but don't know specifics
 please, @ILove Scotch , all youtube videos are vetted for scientific accuracy!
please, @ILove Scotch , all youtube videos are vetted for scientific accuracy!
![]() undecided is certainly not a gospel but the best i can do as of just now in real time.
undecided is certainly not a gospel but the best i can do as of just now in real time.
![]() the mit guy has a whole lecture but that one is LONG.
the mit guy has a whole lecture but that one is LONG.
 I can look at it and get back to you
I can look at it and get back to you
 @tom should ask me, I got tons of tesla battery info lol.. sitting at my desk is Model s module, tesla Roadster module, and i'm building my own 18650/27.... lego module for future designs.
@tom should ask me, I got tons of tesla battery info lol.. sitting at my desk is Model s module, tesla Roadster module, and i'm building my own 18650/27.... lego module for future designs.
 Professors, man, they just go on and on. I should know, that's what I used to do
Professors, man, they just go on and on. I should know, that's what I used to do
 very cool!
very cool!
 those 18650s are still the highest energy density cells Tesla has, iirc. The bigger ones can deliver and take more current, but at a bit of a cost in energy density
those 18650s are still the highest energy density cells Tesla has, iirc. The bigger ones can deliver and take more current, but at a bit of a cost in energy density
 also the 18650s can be cooled better than the bigger cells
also the 18650s can be cooled better than the bigger cells
![]() Flow batteries have that annoying membrane thingy. Though I saw a design of a chlorine flow battery, with water-tetrachlormethane (or water-hydrocarbon) systems, where there is no membrane, and the catholyte-anolyte junction is between immiscible liquids. The water phase is chloride-rich, the organic phase is solvent for chlorine. The junction happens on a layer of activated carbon with titanium-ruthenium oxide catalyst.
Flow batteries have that annoying membrane thingy. Though I saw a design of a chlorine flow battery, with water-tetrachlormethane (or water-hydrocarbon) systems, where there is no membrane, and the catholyte-anolyte junction is between immiscible liquids. The water phase is chloride-rich, the organic phase is solvent for chlorine. The junction happens on a layer of activated carbon with titanium-ruthenium oxide catalyst.
 Is it clear that LFP (Lithium Iron Phosphate) is the safest battery for inside-the-building applications?
Is it clear that LFP (Lithium Iron Phosphate) is the safest battery for inside-the-building applications?
 what's so annoying about a membrane (or a porous separator)
what's so annoying about a membrane (or a porous separator)
 Agreed on FLow, PEM are expensive (to me anyways)
Agreed on FLow, PEM are expensive (to me anyways)
 when I did flow batteries, we used a porous separator
when I did flow batteries, we used a porous separator
 Don't get me started on Nafion. I spent my whole fuel cell career showing them up ;)
Don't get me started on Nafion. I spent my whole fuel cell career showing them up ;)
 @Tom Johnson yeah for existing Li ion types LiFePO4 is pretty safe, can handle higher temps...
@Tom Johnson yeah for existing Li ion types LiFePO4 is pretty safe, can handle higher temps...
![]() Dead Li-ion cells, especially the pouch ones that are easy to disassemble, are a pretty good source of microporous polyolefin membranes. Pretty handy for electrochemistry.
Dead Li-ion cells, especially the pouch ones that are easy to disassemble, are a pretty good source of microporous polyolefin membranes. Pretty handy for electrochemistry.
![]() Is there an alternative to Nafion?
Is there an alternative to Nafion?
 sure. Sulfonated styrene-ethylene
sure. Sulfonated styrene-ethylene
 @Dave Sopchak: you mentioned earlier that there's no solid state battery*.
@Dave Sopchak: you mentioned earlier that there's no solid state battery*.
=>But do you know of a technology that allows a higher density / compactness, for (extreme) miniaturization?
*refering to this:
https://www.electronics-lab.com/meet-the-tiny-100mah-rechargeable-3d-solid-state-batteries-from-iten/
 Thomas- yes. Celgard separators
Thomas- yes. Celgard separators
 hi @Drix the reason solid electrolytes do well in teeny batteries is because the teenier the battery, the less likely the thin solid electrolyte will fail due to volume changes on change in state of charge
hi @Drix the reason solid electrolytes do well in teeny batteries is because the teenier the battery, the less likely the thin solid electrolyte will fail due to volume changes on change in state of charge
 Any developments on radioisotope batteries?
Any developments on radioisotope batteries?
 that's one of the things that's hiding behind the misnomer "solid state". If they are truly solid state, why do things crack and fail, or why do dendrites get through? Solid state devices don't fail that way in normal operation
that's one of the things that's hiding behind the misnomer "solid state". If they are truly solid state, why do things crack and fail, or why do dendrites get through? Solid state devices don't fail that way in normal operation
![]() We did some experiments with laser-induced pyrolysis of kapton for graphene supercaps. With ionic liquid as electrolyte. It worked pretty well, we used the dead-battery separators with success. Also tested briefly for testtube-scale hydrogen production, also works.
We did some experiments with laser-induced pyrolysis of kapton for graphene supercaps. With ionic liquid as electrolyte. It worked pretty well, we used the dead-battery separators with success. Also tested briefly for testtube-scale hydrogen production, also works.
 @kjansky1 What, like the RTGs on the Mars rovers?
@kjansky1 What, like the RTGs on the Mars rovers?
 Speaking of dendrites...any way of breaking them up by vibration?
Speaking of dendrites...any way of breaking them up by vibration?
 a radioisotope battery is a betavoltaic, is that what you mean?
a radioisotope battery is a betavoltaic, is that what you mean?
 or the RTGs?
or the RTGs?
 Yes.
Yes.
![]() Solid state devices love to fail. Electromigration, in chips. Growth of intermetallic phases on bonding. Cracks and delamination on thermal cycling. And on and on and on...
Solid state devices love to fail. Electromigration, in chips. Growth of intermetallic phases on bonding. Cracks and delamination on thermal cycling. And on and on and on...
 yeah Tom also breaking up the solid electrolyte with vibration
yeah Tom also breaking up the solid electrolyte with vibration
 yes and flash memory with ungodly high potential gradients in the gate
yes and flash memory with ungodly high potential gradients in the gate
 RTGs are just fancy thermocouple generators.
RTGs are just fancy thermocouple generators.
 @Dave Sopchak You confused my sarcasm detector.
@Dave Sopchak You confused my sarcasm detector.
 @kjansky1 so you mean betavoltaics
@kjansky1 so you mean betavoltaics

https://hackaday.com/2019/02/08/the-deep-space-energy-crisis-could-soon-be-over/
The Deep Space Energy Crisis Could Soon Be Over
On the face of it, powering most spacecraft would appear to be a straightforward engineering problem. After all, with no clouds to obscure the sun, adorning a satellite with enough solar panels to supply its electrical needs seems like a no-brainer.
 @Dan Maloney Definitely not a battery. ;)
@Dan Maloney Definitely not a battery. ;)
![]() ...and trapping the charge carriers in the oxide and resulting in wear in a similar mechanism as a radiation damage. (random thought, could that damage be annealed by heating the worn flash chips for prolonged period?)
...and trapping the charge carriers in the oxide and resulting in wear in a similar mechanism as a radiation damage. (random thought, could that damage be annealed by heating the worn flash chips for prolonged period?)
 I did a little work with betavoltaics when I was at LLNL
I did a little work with betavoltaics when I was at LLNL
 @Tom Johnson gets it ;)
@Tom Johnson gets it ;)
 Its now one of the 7 things in my head.
Its now one of the 7 things in my head.
 so @kjansky1 betavoltaics (let's say one using tritium)- these things have ungodly high energy densities, but, you can only pull as much current as the betavoltaic is spitting out electrons
so @kjansky1 betavoltaics (let's say one using tritium)- these things have ungodly high energy densities, but, you can only pull as much current as the betavoltaic is spitting out electrons
 so, long use, low current. You could combine one with a battery or a cap to trickle charge it for higher current demands
so, long use, low current. You could combine one with a battery or a cap to trickle charge it for higher current demands
![]() low current does not matter when paired with a supercap for pulsed loads, and used for a sensor that squirts data once per time.
low current does not matter when paired with a supercap for pulsed loads, and used for a sensor that squirts data once per time.
 @Thomas Shaddack great minds....
@Thomas Shaddack great minds....
 Do you have an idea how much power a plutonium RTG that used to be used in a pacemaker, might be able to produce at it's peak
Do you have an idea how much power a plutonium RTG that used to be used in a pacemaker, might be able to produce at it's peak
 wat
wat
 Problem with H3 is its relatively short half life.
Problem with H3 is its relatively short half life.
 they put a plutonium RTG in a pacemaker?!?!?!
they put a plutonium RTG in a pacemaker?!?!?!
 yup
yup
 oy
oy

https://www.medicaldesignandoutsourcing.com/looking-back-the-plutonium-powered-pacemaker/
MEDICAL DESIGN AND OUTSOURCING SAM BRUSCO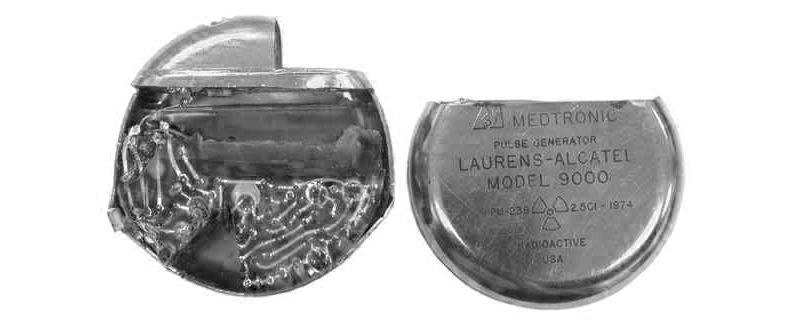
Looking Back: The Plutonium-Powered Pacemaker
The pacemaker has certainly had an interesting journey from its inception-it started as a hand-cranked box that, ironically, scared the life out of people because it leaned a little too close to Frankenstein-esque reanimation. Since then, the smallest model (Medtronic's Micra TPS) is about the size of a large vitamin, with a battery life of up to ten years.
Read this on Medical Design and Outsourcing
 @kjansky1 what kind of time frame were you looking for? 12 year half life not good enough for you I guess
@kjansky1 what kind of time frame were you looking for? 12 year half life not good enough for you I guess
![]() Carbon-14 could be used instead of tritium.
Carbon-14 could be used instead of tritium.
 well Pu is a alpha emitter
well Pu is a alpha emitter
 oh yes we did that at LLNL too
oh yes we did that at LLNL too
 You can use both alpha and gamma emitters with charge induction in a semiconductor junction.
You can use both alpha and gamma emitters with charge induction in a semiconductor junction.
 and when C14 decays, you get nice N14.
and when C14 decays, you get nice N14.
![]() H3 gets even nicer He3. N14 is rather annoyingly common.
H3 gets even nicer He3. N14 is rather annoyingly common.
 I'm not an expert on the alpha/beta/gamma voltaics, but I'm not clueless
I'm not an expert on the alpha/beta/gamma voltaics, but I'm not clueless
 Dan Maloney
Dan Maloney
Discussions
Become a Hackaday.io Member
Create an account to leave a comment. Already have an account? Log In.