In the process of learning how to recycle plastic, I've learned a TON about what plastic actually is, how it's made, what it's made of, and why the different types have the particular (sometimes peculiar) properties that they do.
It's not only fascinating, it's really empowering! For my whole life, plastic has been a material that I have had zero control over. I couldn't make it, I couldn't work with it, and I didn't get to decide what gets made out of it. When I started this journey, basically all I knew about plastic was what most people "know" about plastic:
1) It's made out of oil, and oil is both running out AND killing the world, and you should feel bad about that.
2) It can't be burned, and if it is, it produces a poisonous smoke, and you should feel bad about that.
3) It doesn't decompose, EVER, and it's piling up in the oceans and killing the world, and you should feel bad about that.
4) It's everywhere, it's in everything, and is extremely difficult not to use, and you should feel bad about that. Especially straws.
5) It's made out of toxic chemicals and is probably leaching poison into our water bottle right now, and you should feel bad about that, and probably buy a new water bottle.
Our society's relationship with plastic sums up the feeling I get from our entire industrial economy- Nebulously terrifying, clearly unsustainable, manufactured by a corporate system that is entirely outside of my control, but also still definitely MY FAULT for the fact that it's killing the world.
It's a lot easier to be afraid of things we don't understand, and most people don't really understand plastic. That's why I think the term "Precious Plastic" is so brilliant. In two words it reframes our assumptions about plastic, and refers to it as the precious, abundant, disruptively useful meta-material that it really is.
And of course, all that being said, plastic CAN INDEED be toxic and dangerous. But that's exactly why understanding how, why, and when plastic is toxic is so important- because plastic is far more benign that people often think that it is. When you understand a material, it empowers you to be reasonably cautious about it and to take appropriate safety measures, instead of just being generally afraid of it. Fire is dangerously hot, but it's really, really easy to take the proper precautions to have a fire and not burn yourself. Plastic is the same way. It can be dangerous, but it's fairly easy to avoid danger if you know what to look out for.
SO! This post is going go over all the very-specific ways plastics can be dangerous, so you can take reasonable precautions. It's is accurate to the best of my knowledge, but the best of my knowledge is constantly changing- so revisions, additions, and factual corrections are always welcome. Now, let's revisit over those 5 things people "know" about plastic that I mentioned earlier..
1) Plastic is made out of oil, and oil is both running out AND killing the world, and you should feel bad about that.
So to address this, let's start with something a little more basic- what even is plastic? There are many different types of plastics, and they all have different chemical and physical properties. However, all plastics are polymers, which just means that they are long, repeating molecular chains of a base-molecule, called a monomer. Poly-ethylene, for example, is just a long repeating chain (polymer) of the ethylene monomer.
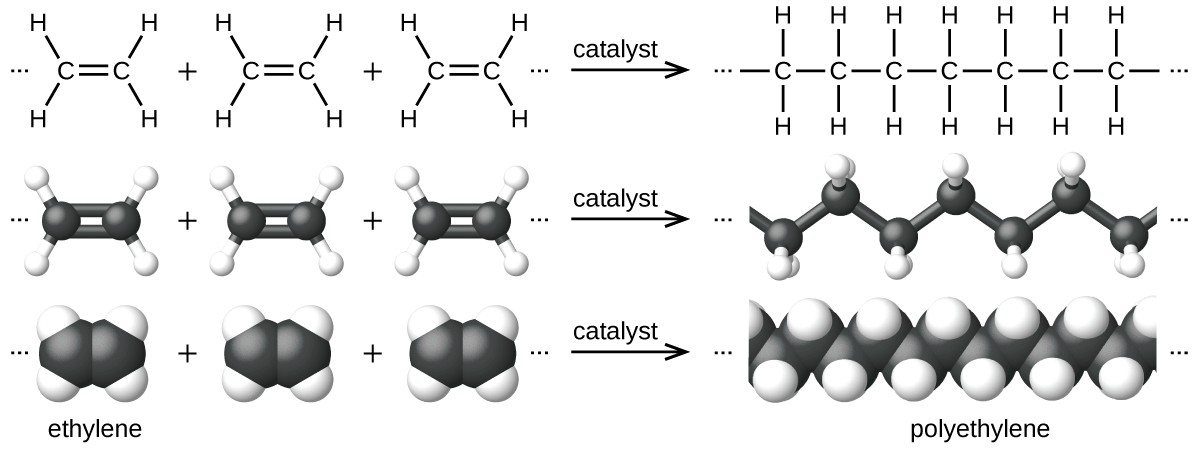
But where did that ethylene come from in the first place? Most people know that most plastics are made from oil, but are a little sketchy on exactly how. So here's a quick recap- crude oil is ancient organic material, mostly plants (basically fossilized sunshine), that were not fully decomposed by animals, fungi, and bacteria before being buried deep underground by sedimentation and/or plate tectonics, which cooked the material under heat and pressure in such a way that the organic molecules making up the proteins and DNA and tissues of the formerly-living organic material sort of agglomerated into large, more-complex organic molecules (to put it overly-simply, this happens because Carbon is really f*cking sticky, molecularly speaking, and that's part of why we're here having this conversation at all).
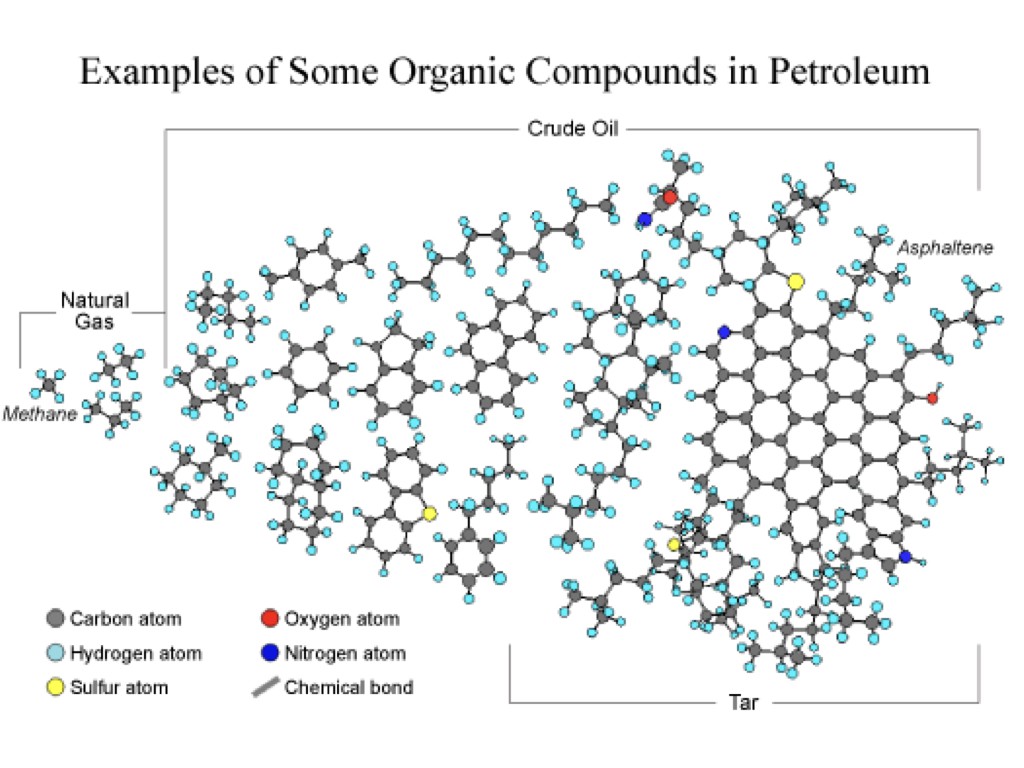
"Crude oil" is just a catch-all term for oil as it comes out of the ground- which is just a mixture of hydrocarbons (with a few other bits in there too, sulfur, nitrogen, oxygen, although in much smaller amounts). That's why crude oil can have "flavors" like "light" and "sweet". The lighter the crude is, the smaller the hydrocarbon molecules it contains are, on average, and that makes it clearer and less viscous. The "sweeter" crude is, the less sulfur and other compounds it contains, making it easier to refine into gasoline. Saudi Crude oil is particularly light and sweet.
Fractional Distillation, which is a primary process in refining (sorting) crude oil into it's molecular fractions, is a very simple process in theory, and only slightly less simple in practice. Crude oil is heated up in a furnace without oxygen, and the heat causes the big molecules to break apart into smaller molecules, which boil off into a gaseous smoke, and that smoke escapes up a distillation column, which cools the gas and causes different hydrocarbons of different molecular weights (sizes) to condense out of the gas stream into liquids. The heavier molecules distill out first, at higher temps, they smaller molecules distill out at lower temps.
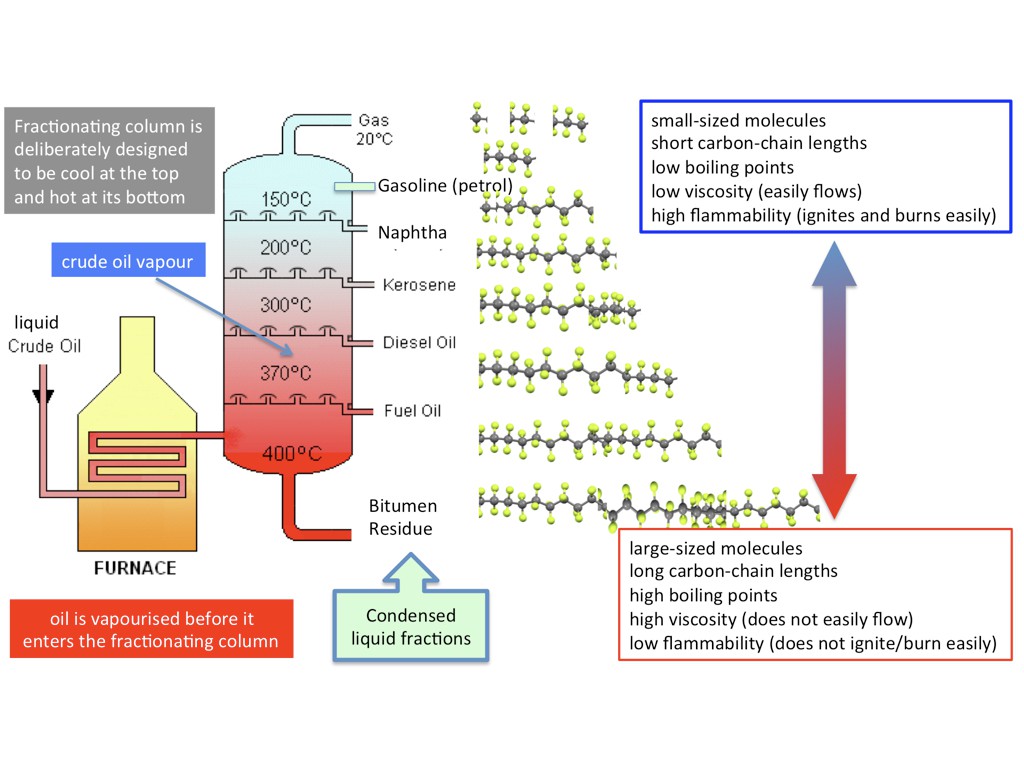
The gas left over is used to power the refinery. If this process sounds familiar, it's because this is exactly what the Metabolizer does, just with trash and biomass as the hydrocarbon source instead of crude oil.

Ethylene, and other small hydrocarbons used to manufacture plastics, are typically made from steam-cracking oil and gas into smaller components, and then distilling/sorting them by type.
That's how plastics relate to oil. TL;DR- Crude oil is broken down into small molecular bits with heat, condensed and sorted by molecular weight, refined into single monomers, just as ethylene, and then catalyzed into long chains. Onto the next one!
2) Plastic can't be burned, and if it is, it produces a poisonous smoke, and you should feel bad about that.
It's true that all plastics can be toxic if burned- particularly if burned on an open fire, which leads to uneven, low-heat burning. This causes the polymers to break apart, but not ALL THE WAY apart, creating smaller hydrocarbons, some of which can be toxic.
All plastics are composed primarily of the elements Hydrogen and Carbon. Most of the common plastics, including ABS, PS, PP, LDPE, and HDPE contain ONLY Hydrogen and Carbon. If burned on an open fire, they can create molecularly toxic compounds, such as benzene:
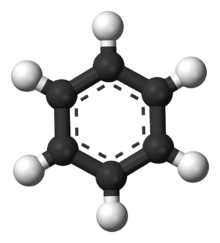
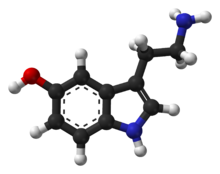
Compounds like benzene are carcinogenic and generally mess with your body, because they are they look and sometimes act like things your body uses all the time, for example, seratonin, which has a benzene backbone:
But contact with these compounds can be avoided by either burning plastic in a reactor that gets so hot that everything is burned off, and/or condenses these compounds and reflows them back into the reactor where they are burned up completely and add energy to the system. If you burned these kinds of plastics ALLLLL the way down, you'll get a flammable mixture of Carbon Monoxide and Hydrogen, often called Syngas, which is an odorless, colorless, flammable gas- when ignited, the Hydrogen reacts with Oxygen in the air to produce Water Vapor (H20) and Carbon Monoxide reacts with Oxygen to produce Carbon Dioxide (CO2)- both of which are 100% non-toxic bioavailable compounds that the biosphere knows how to process.
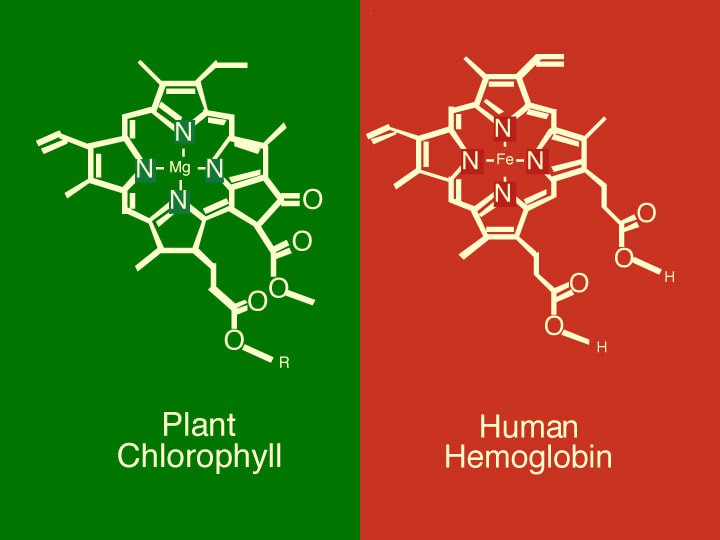
Carbon Monoxide can be toxic if it is breathed in large quantities- it binds to the Hemoglobin in your blood, but doesn't let go the way that Oxygen does. Fascinating side note: Did you know that the Hemoglobin in your blood that carry oxygen to your cells and the Chlorophyll in plants that acts as a photo-catalyst for sunlight, are very-nearly identical molecules? The only fundamental difference is that Chlorophyll has a Magnesium atom at it's core, and Hemoglobin have an Iron atom at it's core.
Anyway, if you breath too much CO, it will prevent your blood from carrying oxygen. However, this is easily avoided by using CO sensors when working with syngas, working in a well ventilated area - the same as you would with a Charcoal BBQ. If done properly, with the proper precautions and safety measures, plastics like ABS, PS, PP, LDPE, and HDPE- that is, the vast majority of all waste plastics- can all be chemically reduced to CO2 and Water Vapor, releasing a large amount of energy in the process.
There are some plastics you need to be more careful with, as some plastics have other elements incorporated into their structure in addition to Hydrogen and Carbon. For example, PET (polyethylene terephthalate) and Polycarbonate, both have some Oxygen in there too. Other, less common plastics, also contain other, more exotic and potentially-harmful elements as well. Teflon (PTFE- Polytetrafluoroethylene), for example, is a "Fluoropolymer" and contains the element Fluorine. The most notable/common example in the waste stream is PVC (#3, Poly-vinyl Chloride), which contains the element Chlorine.
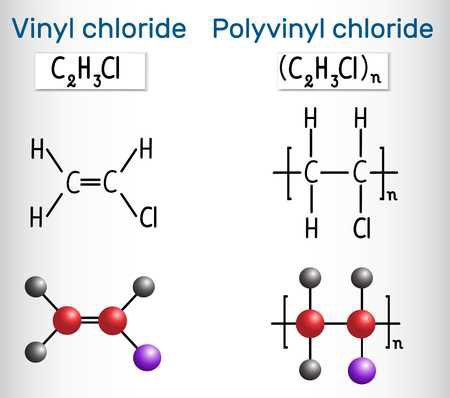
PVC is nearly chemically identical to Polyethylene, but it has a single Chlorine molecule in the place of one of the Hydrogen atoms. When PVC is burned, the Hydrogen and Chlorine are released, and they recombine into Hydrochloric Acid- which is dangerous and highly corrosive. Burning PVC It can also produce Dioxins, which are also toxic. It's best to just avoid ever burning PVC- which is easy to do, as things made of it are fairly easy to identify. The easy approach is to just never burn waste if you aren't sure what it is.
PVC wastes can be repurposed or recast into new shapes or building blocks, without releasing HCl. And bubbling a gas containing HCl through water containing Sodium Hydroxide (NaOH, AKA Lye, which is a widely available component of wood-ash) causes the HCl to react with the NaOH to produce NaCl (AKA table salt) and Water (H20). So while burning PVC is not a good idea and outside the scope of this project (for now), it's quite feasible, even on a small scale, to design systems that can entirely scrub it from the waste stream.
3) Plastic doesn't decompose, EVER, and it's piling up in the oceans and killing the world, and you should feel bad about that.
It's true that plastics in nature take a long time (1000s of years, potentially) to decompose, and when they do, they often just break apart into other, smaller, still-toxic compounds. But that doesn't mean that plastic is forever, which is what most people think. We just need to help it along, and a great way to start is to stop throwing it in the oceans. If we had a real viable strategy to collect, recycle, decompose, and process plastics, we could use them in a renewable way, benefitting from their useful qualities, and designing systems that entirely avoid or manage their negative qualities.
The catch is- that's on us. We made the plastic, and very few living things can break it down again (yet), so it's our responsibility to be smart about how we use them, and what we do when we're done with them.
4) Plastic is everywhere, it's in everything, and it's extremely difficult not to use, and you should feel bad about that. Especially straws.
While single-use plastic is poor way to use plastic, it's not necessarily the problem. The problem is an industrial infrastructure that invests heavily in (and profits heavily from) the production of plastics, and puts little to no effort into creating viable systems to actually recover and recycle them in a real way (not in a sort, palletize, and ship them to china kind of way). If we have a process in place for actually dealing with plastics, then we don't have to feel bad about using them. And having to do that, to treating plastic as a precious material, often changes the ways that we choose to use them.
5) Plastic is made out of toxic chemicals and is probably leaching poison into your water bottle right now, and you should feel bad about that, and probably buy a new water bottle.
The whole thing about water bottles leaching BPA comes from water bottles that are made out of Polycarbonate (AKA PC, Lexan, Makrolon), which is polymer made up partially of the monomer Bisphenol A:

Bisphenol A has an organic structure that is similar enough to Estradiol - a human hormone produced by the endocrine system- that it can disrupt normal functioning of cells. But Bisphenol A is present ONLY in Polycarbonate, which is a comparatively uncommon plastic in the waste stream, and is only really a problem if you're drinking out of it. The simple solution here is- don't drink out of it! There are plenty of other materials to drink out of (I like stainless steel) and that way we can save polycarbonate for the things it's really good at, like stopping bullets or building greenhouses (or building bullet-proof greenhouses...)
In conclusion:
Be cautious, be smart, be prepared, but don't be afraid of plastics. Like other dangerous things that we interact with every day, like cars, stoves, or the Internet, they can be used in a way minimizes or eliminates their negative potential. All it requires is care, a willingness to learn, and a belief that we can learn to understand problems and design solutions to deal with them.
Plastics aren't bad, and humans aren't bad for making them. Plastics are complicated, and humans have, to-date, been too careless with them. But it's worth noting that all the plastics that we know and use today have only been around for 100 years or less. I think we can be forgiven for not knowing how to deal with them until just now, IF we start learning how to deal with them, right now.
 Sam Smith
Sam Smith
Discussions
Become a Hackaday.io Member
Create an account to leave a comment. Already have an account? Log In.