"What a waste," you may say "those Joules could have been photons, man!" But they were sacrificed in the name of knowledge...
So, I did the first experiment with supercapacitors and spit tonight. Not particularly rigorous but still interesting.
Setup and method
The control was the same as for the coin cell experiment: a 5g acrylic jar with spit in it.
The test setup started with a pair of 3F 2.7V Cooper Bussman EDLCs wired in series. They were charged through a 20 Ohm resistor to 4.9V.
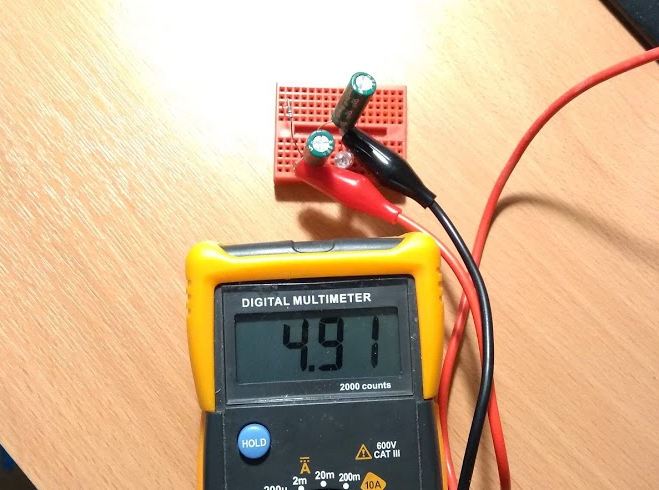
The part of the circuit which was submerged in spit was an attempt to realistically recreate what a badge might look like (as far as electrolysis of spit is concerned). I used a piece of protoboard with some spare LED legs that had been clipped off as extensions to six 0.1" header pins.
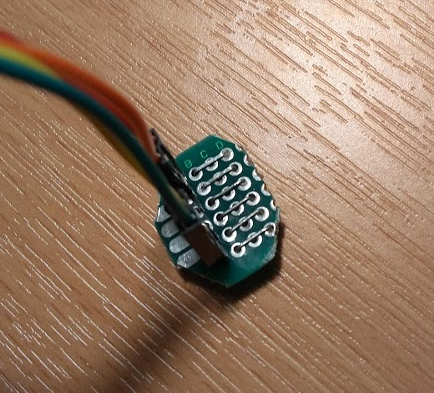
The furthest pin was connected with the red wire to the 4.9V leg of the higer voltage supercap. The next furthest pin was connected (orange wire) to the ~2.45V middle where the two supercaps connected to each other. This represented one capacitor pair of pins being submerged. Then I left a gap of two pins to represent the fact there would be spacing between the legs of the pair of supercaps. Then the second nearest was connected to the 2.45V midpoint (yellow wire) and the nearest pin was connected to the negative terminal (green wire) of the lower potential supercap / ground.
Here's the whole thing fizzing* away once I submerged the "probe":

*it didn't fizz but I'm sure there weren't that many bubbles there when I made the spit sample...
Results
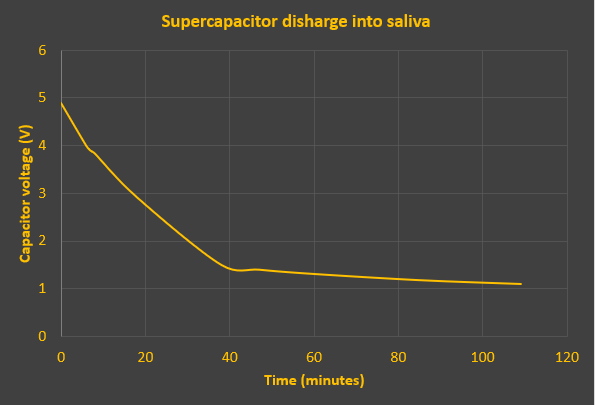
Here's the discharge curve. Experiment stopped at 109 minutes, slightly short of the previous experiment. Final voltage was 1.1V. The irregularity of readings (to fit around my dinner time) would embarrass my science teachers.
And here's the litmus test result:

As you can probably guess, the control sample is at the top and the supercap sample is below. I would say we're definitely looking less caustic than the coin cell, at around pH 10 or so, but I still wouldn't want this lodged in my gullet!
Conclusion
I was slightly surprised that, relative to a CR2032, the increased spacing of the pins and wires, combined with a lower total energy stored, didn't lead to a less caustic outcome than the results show. This should prove for an interesting test of the hypothesis that increasing the shortest electrical path between the anode and cathode in coin cells would significantly reduce the pH of the resultant hydrolysed saliva.
The really nice thing about supercaps, though, is that they can be soldered into the PCB of a badge and left there, being charged in place and never (in all likelihood) needing replacing. If your badge is larger than a CR2032 and you can run it on supercaps, you have a decent chance of being safer purely because your badge is harder to swallow than a cell on its own.
For those who are wondering, the supercaps I used are a nice size if you are thinking about badge mounting. At 20mm long and 8mm diameter, they feel like a roughly AAA replacement size (albeit a little longer if you have them end to end) but with more options to place around a board. Two easily fit on a 1" square PCB, laid flat. Just think about whether you need to glue or otherwise fix the non-leaded ends down mechanically. Wouldn't want them being pulled off the PCB.
I'll leave it to someone else to provide the nice calculations on how many Joules were used to hydrolyse the saliva in this experiment.
 Simon Merrett
Simon Merrett
Discussions
Become a Hackaday.io Member
Create an account to leave a comment. Already have an account? Log In.