Chris Justice of Engenious Design, a medical product development firm, recently volunteered some valuable insight into the FDA approval process for medical devices in the United States. While the OpenFluidWarmer project is unlikely to pursue FDA approval because of how expensive the process is, it is a goal of this project to meet as many FDA approval requirements as possible.
A good first step of understanding the FDA approval process is to identify the product code for the type of medical device being evaluated. This search is done through the FDA Product Code Classification Database. For IV fluid warmer designs like the OpenFluidWarmer that to not use electromagnetic radiation (radio waves or microwaves) to warm the IV fluid, the product code is BSB. We also learn that device class is II and submission type is 510(k) for BSB medical devices. The image below is the database search result.
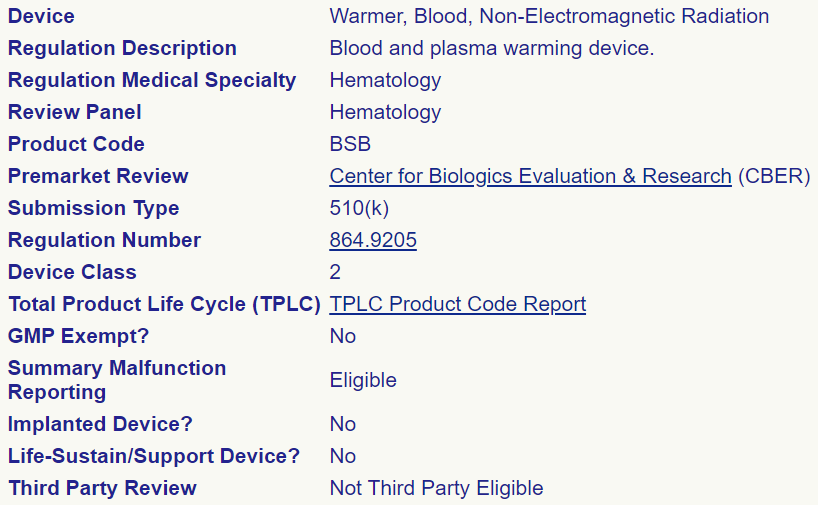
In addition, with knowledge of the product code, we can look up the competitor BSB devices that have already received FDA approval.
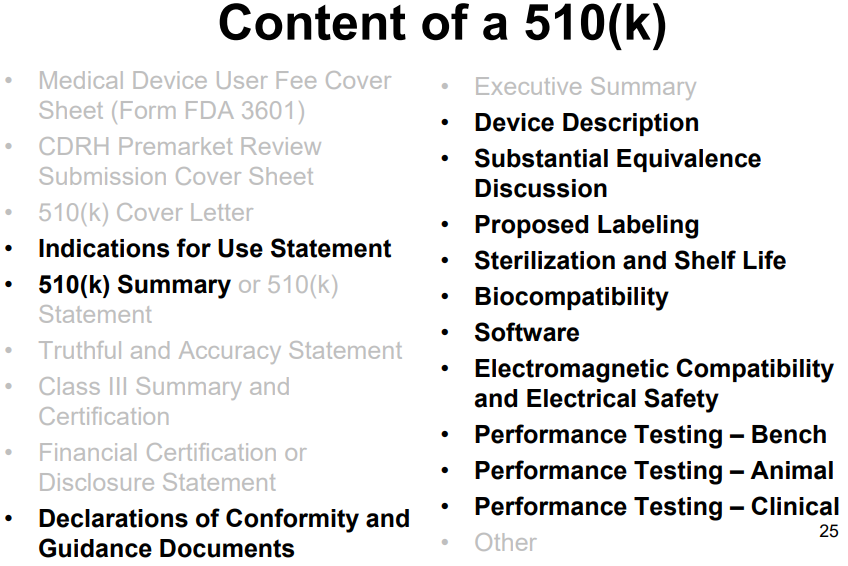
"A 510(k) is a premarket submission made to FDA to demonstrate that the device to be marketed is as safe and effective, that is, substantially equivalent, to a [existing] legally marketed device." The content of a 510(k) submission is summarized in the image below. More information on the premarket notification 510(k) program can be found here and here.
The following standards/regulations also apply to the OpenFluidWarmer device:
- IEC 60601 design standard - "a series of technical standards for the safety and essential performance of medical electrical equipment"
- 21 CFR 820 US FDA quality management regulations - "requirements in this part govern the methods used in, and the facilities and controls used for, the design, manufacture, packaging, labeling, storage, installation, and servicing of all finished devices intended for human use."
- ISO 13485 international quality management regulations, "specifies requirements for a quality management system where an organization needs to demonstrate its ability to provide medical devices and related services that consistently meet customer and applicable regulatory requirements."
More work is required to fully understand all the implications of everything discussed in this log on the final OpenFluidWarmer design, but I see just having a clear understanding of what medical device standards and regulations to follow as a big step forward.
 John Opsahl
John Opsahl
Discussions
Become a Hackaday.io Member
Create an account to leave a comment. Already have an account? Log In.