A key concept at the heart of this project is that of color temperature. As such, allow for this short introduction to the history of the concept. Future entries may further elaborate on the complexities associated with this metric, but the following should provide at least a basic introduction to how this terminology has become a core component of the discussion when talking about light sources.
Electric light sources had existed in different forms prior to the commercialization of the tungsten filament incandescent light bulb around the start of the 20th century. However, the continuing presence of incandescent bulbs over a century after their initial popularization underscores the importance of understanding their basic operation and critical drawbacks. The basic construction of traditional light bulbs incorporates a glass enclosure which contains a filament wire connected to electrical contacts in a base. The glass enclosure is evacuated and typically filled with an inert gas. The filament materials are selected and constructed to provide a targeted amount of resistance when connected to a source of electricity. The most common material associated with filament bulbs is tungsten, but early models used carbon filaments. The result is that when connected to an electrical source current passes through the filament such that it is heated to a target temperature. Once heated the filament will glow, or incandesce, per a relationship between an object’s associated temperature and the total energy of radiation emitted as well as the color of visible light emitted. At lower temperatures, a source emits relatively low levels of total energy with a peak of the energy being in the infrared and red parts of the spectrum1. This is generally perceived as glowing red hot. However, as the object is heated up more energy overall is emitted and the spectrum includes more components of the visible spectrum (generally composed of wavelengths of light between 400 nm to 700 nm). An object reaching these higher temperatures is generally perceived as glowing white hot. Color temperature defines the wavelength at which the peak energy is emitted and as such is directly associated with the relative balances of energy for each of the wavelengths of visible light. Note in the figure below how only a small section of the wavelengths (marked on the left) include the visible light spectrum.
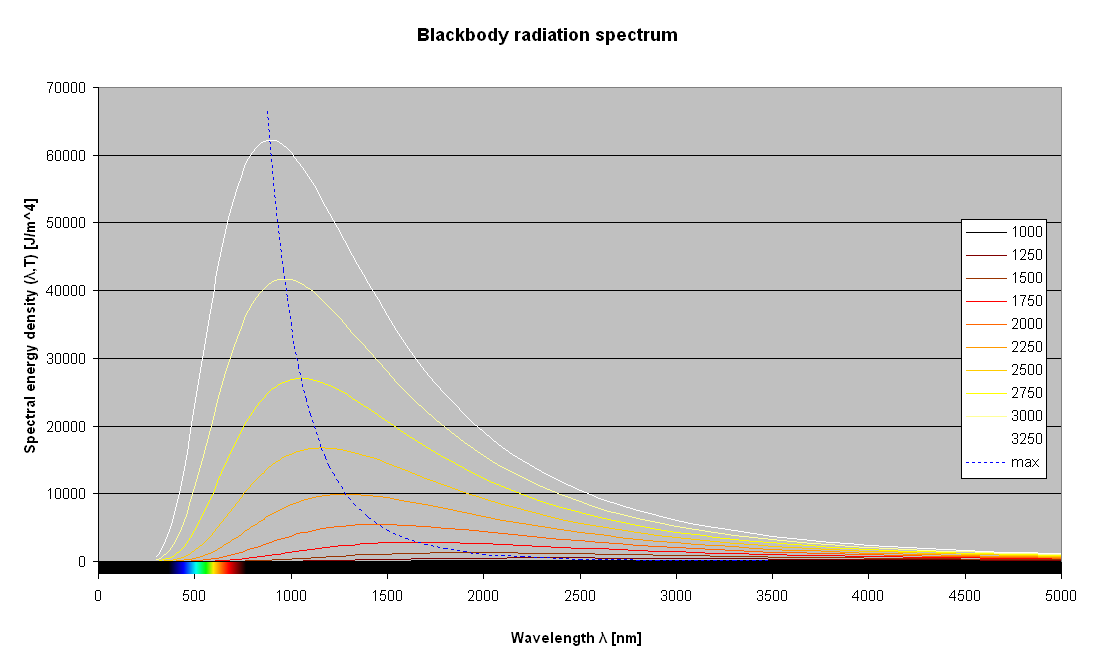 |
| The energy curve for each temperature demonstrates that invisible infrared wavelengths (> 700 nm) represent most emitted radiation for incandescent sources. Visible light wavelentghs are highlighted by the color rainbow on the left. The peak energy wavelength decreases as the temperature of the source increases. Diagram excerpted from Iacopo Giangrandi's "Black Body Radiation and Color Temperature."1 |
The primary advantage of such a light source is that it is comparable to the light emitted from the sun. Although not seemingly related, both an incandescent light bulb2 and the sun generate light though heating3. The spectrum of light produced includes the continuous spectrum of light wavelengths across the entire visible spectrum. The significant disadvantage of such sources is that radiation generated typically includes a significant component of wavelengths outside of the visible light spectrum. In the case of incandescent bulbs, although essentially 100% of input power translates to output energy only 6% of that energy is within the visible spectrum4. A disproportionate amount of power input is converted into invisible infrared energy which is perceived as heat radiating from the bulb. The efficacy of a given source is expressed in the luminous flux, a measure of the power of light radiation as perceived by the human eye, per watt. An ideal source defined based upon the 5800-degree Kelvin color temperature spectrum associated with the midday sun, but restricted to only the visible light spectrum, would possess an efficacy of 250 lumens per watt. That is reduced to 15 lumens per watt in the incandescent example presented earlier (and this is only under ideal conditions with no real-world losses). With the filament typically heated to around 2800 degrees Kelvin the tungsten incandescent lamp is more efficient at producing heat than visible light.
Of course, newer light sources introduce some significant changes in how light is generated. As such, the impacts of those changes to this metric are worth further discussion... for a later post!
1 Giangrandi, Iacopo. “Black Body Radiation and Color Temperature.” Giagrandi.ch, www.giangrandi.ch/optics/blackbody/blackbody.shtml.
2 Orzel, Chad. “The Surprisingly Complicated Physics of a Light Bulb.” Forbes, Forbes Magazine, 21 May. 2015, www.forbes.com/sites/coxbusiness/2017/08/01/how-to-serve-clients-when-workers-arent-at-work/#4c9974345cfe.
3 Bahcall, John N. “How the Sun Shines.” Nobelprize.org, Nobel Media, 29 June 2000, www.nobelprize.org/nobel_prizes/themes/physics/fusion/.
4 Murphy, Tom. “Maximum Efficiency of White Light.” 31 July 2011, tmurphy.physics.ucsd.edu/.
 Jon
Jon
Discussions
Become a Hackaday.io Member
Create an account to leave a comment. Already have an account? Log In.