Background
A brief overview of microfluidics
Microfluidics are often hailed as a revolution in biology, akin to how the transistor and ICs revolutionized electronics and computing. Chips can be designed to run many different kinds of lab experiments, with the advantage that they use much lower volumes of reagents, and are usually faster and more accurate than "conventional" assays.
Some common applications of microfluidics include diagnostics (e.g. detection of disease markers from a single drop of blood), drug screening, DNA sequencing and cell culture. This Wikipedia article gives a good introduction to the field.
Practically, fluids have to be pushed around the chips somehow. There are several ways to do this. One is to use passive flow, which relies on the affinity of the fluid and the substrate to pull the fluid along. This is used for example in glucose test strips, which are basically strips of paper that wick a drop of blood to an electrochemical sensor.
Another -- very cool -- way of moving fluids around a chip is electrowetting, which in a nutshell consists of applying a high voltage to electrodes on the surface of the chip to make a given electrode more hydrophilic, thus allowing you to pull a droplet of water along. A great example of this is OpenDrop.
Finally, fluids can be pushed around by air pressure. This is usually accomplished either with a syringe pump, which establishes a given flow rate, or with a source such as a pump and pressure regulator, which impose a given pressure.
There are of course other methods, but these are the most common. Without going into too many details, it is this last method -- pressure-driven flow -- which we need to operate our microfluidic chips. In our lab, we use these chips to culture cells in a way that simulates "real-life" tissue. This allows us to carry out experiments to better understand how cells interact in vivo, while keeping them in a highly controlled environment, and allowing us to run many experiments in parallel with relatively little manual labor.
If you are interested in learning more about microfluidics and how they can be applied to biology, here is a great introductory lecture on the subject. The type of chips that we use (and is mentioned in that lecture) are constructed of two layers of PDMS. These are great for our application since they make it easy to integrate valves into our designs, allowing us to route fluids to specific parts of a chip, and run many different experiments in parallel on a single chip.
Control systems for PDMS chips
In short, to operate our chips, we need:
- A positive pressure source
- A vacuum source
- A way to regulate pressure (at three different pressure levels, including vacuum)
- Valves to switch pressure on & off on up to 32 lines independently
- A way to automate everything
While this is specific to our application, it should be noted that most of these requirements (especially numbers 1 and 3, and to some extent 5) are common to every lab using similar microfluidic chips, so this project could benefit many other labs and even DIY biologists.
Now, there are commercial solutions for controlling microfluidics. You get either a modular system with separate pressure sources, valves and so on, or a nice all-in-one unit. Unfortunately, all of these tend to be rather expensive (5-10k USD or more), and not always suited to one's exact application. For example, you might find commercial controllers with 16 valves; if you need just a few more, then you have to shell out an extra few thousand dollars for a second valve control module.
Therefore, many labs go for a more DIY approach.
Typically, the pressure sources are either central compressed air for the fancy labs, or large pumps and tanks for the plumbing-impaired labs. The pressure is then adjusted using a manual regulator and the air is split by a manifold to the many different lines, each of which is controlled either with manual valves, or solenoid valves controlled by a PC or microcontroller.
A neater solution is to integrate everything into one box: pumps, pressure regulators, valves, and a microcontroller to control everything. This is what this project is about: the goal is to have an all-in-one control system that is as good or better than existing solutions, while being cheaper and open source.
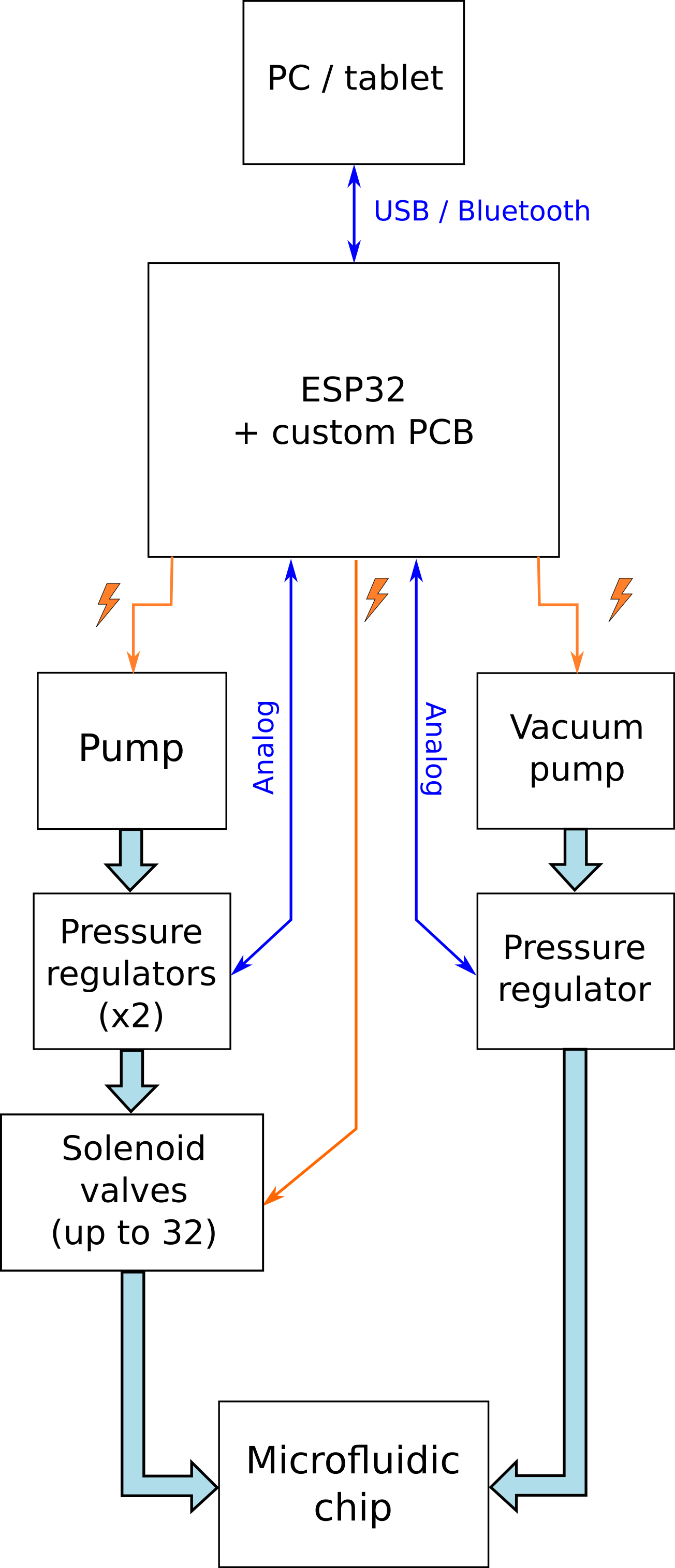
Here is a block diagram of the system. Thin arrows represent data or power transmission; thick lines indicate air or liquid flow:
Goals
The project was already well underway at the time of writing this and so some of these goals have been completed already. Roughly, the main goals of the project are:
[x] Design a "shield" PCB for an ESP32 development board to control 32 solenoid valves, 2 pumps and 3 pressure regulators
[x] Assemble & test hardware
[x] Write ESP32 code: must allow control of all components via serial communication (USB at first, eventually Bluetooth)
[~] Write PC code: must show status of components, allow manual control and eventually automated control [in progress]
[ ] Design and fabricate an enclosure
Time permitting, future versions should include:
- Integrated pressure regulators, to reduce cost
- ESP32 module, rather than a development board
For more details on the choice of components and the design of the system, I recommend reading through the project logs in chronological order
About the project
The microfluidics control system is developed in the Senyo Lab at Case Western Reserve University in Cleveland, OH, USA. Version 0.1, the first prototype, was developed in the LBNC at EPFL in Lausanne, Switzerland. From the beginning, the designs were meant to be open-sourced, and now that I have had time to properly document the system, I am finally publishing all the details.
Version 0.1 was intended as a prototype for a point-of-care diagnostics system, while version 1 is going to be used mainly for cell culture experiments. However, it could be used with any pressure-driven microfluidic chip, PDMS or otherwise.
This is a bit of a side-project and so the goal wasn't to go all-out and make a perfect system with version 1. Some compromises had to be made to keep development time low, such as the use of external pressure regulators (which account for more than half of the cost of the entire system). Hopefully, there will be future revisions of the design that improve on, and fix some issues with v1.
 Craig Watson
Craig Watson