Why this might work when other attempts have failed
Several readers pointed out that others have tried to fixate Nitrogen using ultrasonics, and none succeeded.
Supeno's paper "Sonochemical Fixation of Nitrogen" reviews the previous attempts and has a good explanation of the difficulties involved. If you're interested, it's an easy read and provides good background information for this project.
Of course you don't do what others have done and expect different results. For different results, you have to do something no one has tried. Preferably with a rationale for why the differences are important, and how they might lead to success.
So here at last is the secret sauce: what I intend to do that is different from previous attempts!
Difference 1: Focused ultrasonics
Previous attempts used ultrasonics at low power density. More specifically, previous attempts made no attempt to focus the ultrasonic energy to high density.
Supeno experimented with a tuned chamber that, as far as I can tell, generates standing [planar] waves. The energy within the chamber is the transducer power divided by the base area times the Q of the tuned system.
He used roughly(*) 50 watts from a transducer 2.125" in diameter, which is 2.2 watts/cm^2, which is not a high energy density. His paper doesn't list the tuned chamber specs, but if the Q is 20 then the energy density would still be only 44 watts/cm^2.
(*) Various depending on the experiment, but in this range.
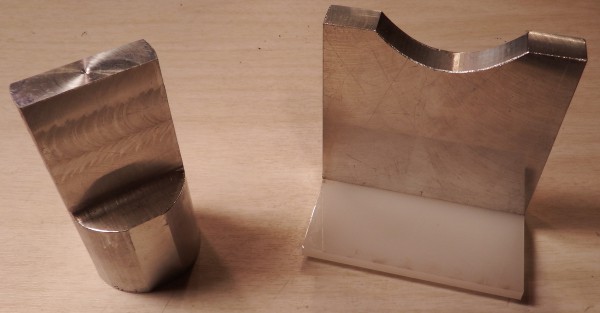
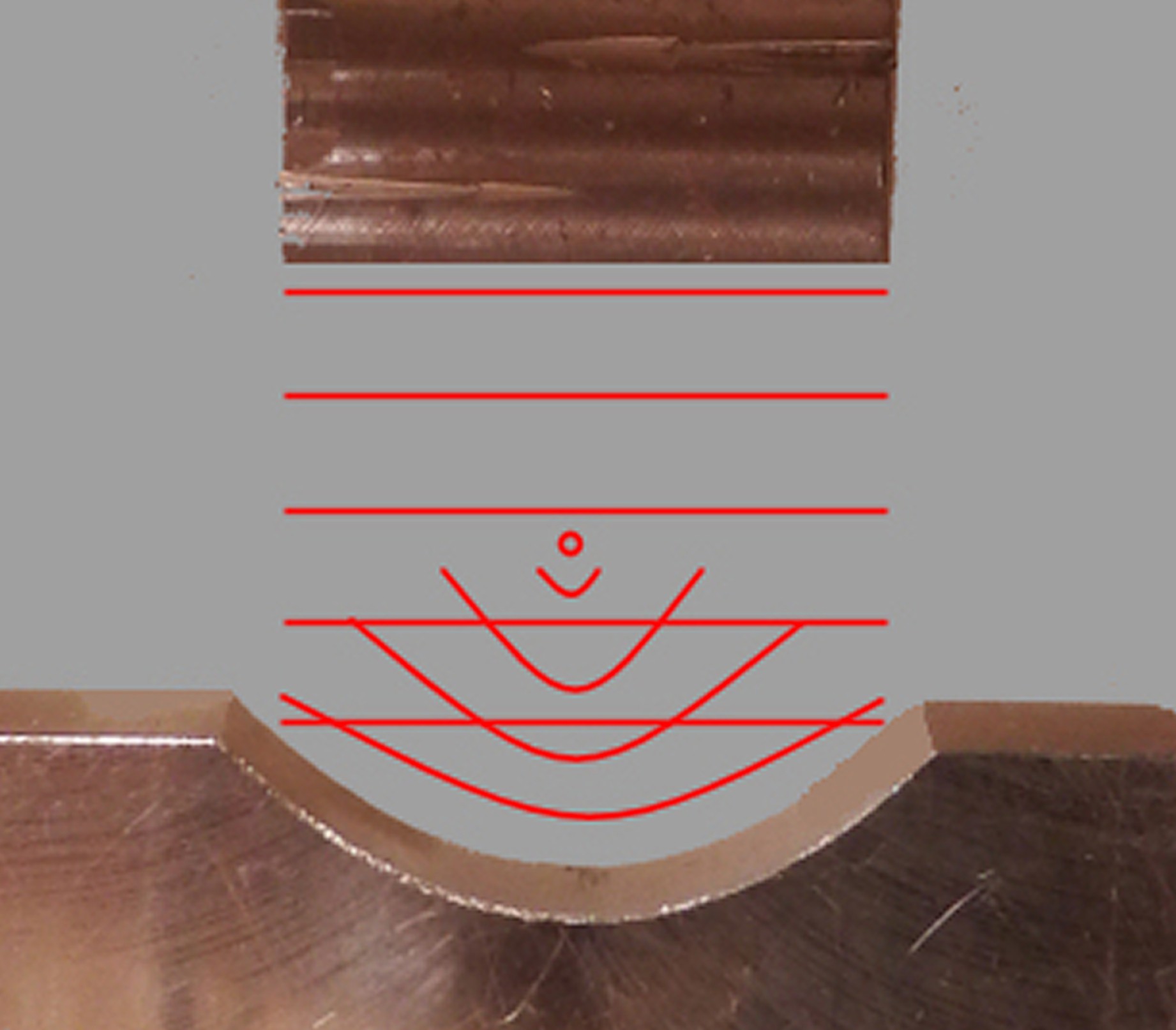
For my experiments, I've milled a rectangular end horn and a parabolic reflector anvil.
In theory, the rectangular horn should generate plane waves which will be concentrated by the parabolic reflector. This should produce enormous energy densities and pressures, which are different experimental conditions from the other attempts.
Difference 2: Directly sonicating the gas
Previous attempts used Nitrogen and Hydrogen dissolved in water. Specifically, in previous attempts Nitrogen and Hydrogen were bubbled through water using an air stone, and sonicated.
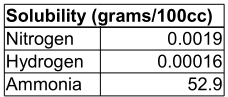
Hydrogen and Nitrogen are largely insoluble in water, so one would expect such a system to have a poor yield.
...but note that previous experiments did produce tiny amounts of ammonia. That's important!
It takes 3 moles of Hydrogen to produce 1 mole of ammonia, so dividing the Hydrogen solubility by 3 tells us that the most ammonia we can get from 100cc of water using this method is about 50 micromoles(*).
Further, the molar proportion of dissolved Nitrogen to Hydrogen is 0.8:1, which is nowhere near the ammonia ratio of 1:3. This reduces the maximum yield: with less Hydrogen available than needed, fewer activated Nitrogens will encounter Hydrogens to form ammonia.
Supeno experimented on 100cc batches and produced around 5 nanomoles of ammonia(**). Perhaps ultrasonic production of ammonia might work with a different setup.
(*) Gloss gloss, such as continually replenished gases and not accounting for sonification time.
(**) Various, depending on experiment, see paper.

My system uses a capillary tube to hold a small bubble of gas (mixed to molar proportions) at the focal point of the parabolic reflector.
The hope is that focused ultrasound will compress the bubble to high pressures, generated ammonia will dissolve into the surrounding water, the bubble will shrink, and gas will be replaced as needed by a mass flow controller.
For now I'll manually control the mass flow via serial port, but if ammonia is generated it seems reasonable to automate this using a webcam and OpenCV.
Modification of parameters
Experimentation is a form of search, and to increase the chances of finding something you need a wide search area.
With this setup I can vary the ultrasonic power delivered to the bubble, the size of the bubble (a little), the temperature of the water, and the gas mixture (proportions, or adding a buffer gas such as Argon). I can also dissolve things in the water which might catalyze the reaction, and I can try to make other compounds such as oxides of Nitrogen.
My plan is to take measurements under various conditions, look for trends in ammonia formation (if any), and zero in on the most effective experimental regime. At that point I can estimate the efficiency and compare to the Haber process.
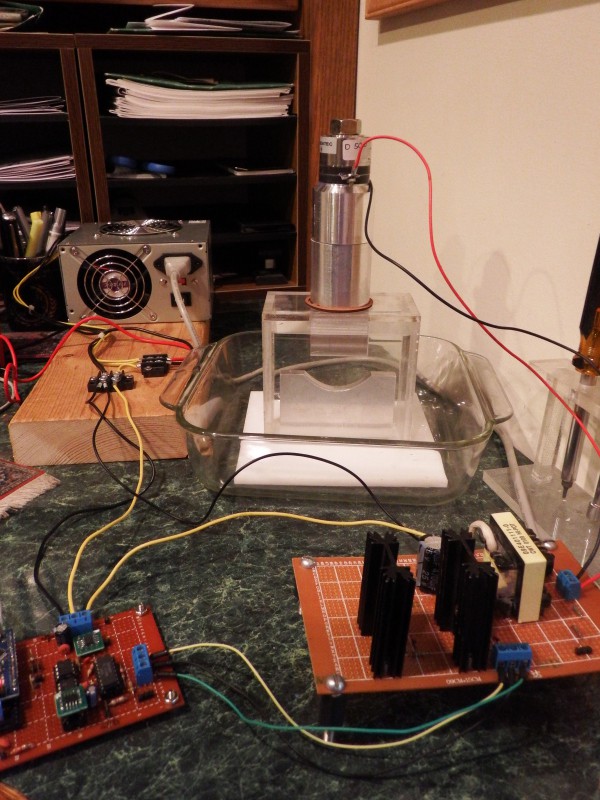
Video of the system in action
Here's a video of the reaction chamber showing horn and anvil. It doesn't have a capillary tubing mount (under construction), but I have an air bell for collecting gas, Hydrogen and Nitrogen generators, and a mass flow controller.
This the same effect as the 2KW cavitation mentioned in the a previously mentioned video, at a smaller scale.
The cloudy wisps extending from the bottom of the horn are flows of cavitation bubbles. I think. Power output around 50 watts, through a cylindrical horn sculpted to a 44x12 mm rectangular(ish) end.

 Peter Walsh
Peter Walsh
Discussions
Become a Hackaday.io Member
Create an account to leave a comment. Already have an account? Log In.
Great questions and answers, can't wait to see how this works! Something I just noticed on the wikipedia page for the Haber process - do you need the iron catalyst for this to work? I guess maybe you are thinking this will only complicate the proof of concept experiment, and could be a future improvement if the first tests appear to work.
Are you sure? yes | no
You are correct about the reflector, I should be more clear in the description.
The system can be used for several purposes, including mixing and general cavitation. The comment about smaller ends was addressing those other uses - using a smaller tip to get more energy density. For example, faster mixing or "hotter" plastic welding. Nitrogen fixation addresses the energy density with the reflector. (I'll update the description.)
As to oscillating bubbles, as far as I can tell no one has done the experiment in this way so yes, it may be a problem.
I've been avoiding chemistry and physics explanations because the readership seems more comfortable with electronics, but a lot of theory and studies have been done about bubble cavitation.
Background 1: Sonoluminescence in water comes from a trapped air bubble. It has to be formed and moved into place, but it's an actual bubble and not a vacuum void. (viz: Wikipedia: https://en.wikipedia.org/wiki/Mechanism_of_sonoluminescence)
Background 2: In my setup, a capillary tube will hold a single bubble at the focal point of the reflector.
Firstly, will a compressed bubble simply retreat into the tube?
Research indicates that it will not. Changes in pressure happen at the speed of sound, and the speed of sound in water is much higher than in air (1482 versus 380 m/s). Informally, the water should compress the air faster than it can get out of the way. I can also enhance the effect by using a tiny tube (for example: a syringe tip) relative to the size of the bubble.
Secondly, will converted ammonia dissolve into the water?
Studies of cavitation indicate that the high pressures occur at the water/air boundary. This is consistent with the air being compressed faster than it can get out of the way. I expect created Ammonia will be near the surface of the compressed bubble, and have close or direct contact with the water/air interface where it can be absorbed.
So overall I'm hoping that a fixed bubble will shrink as converted ammonia is absorbed into the water. When this happens, the system will supply more gas to account for the difference.
...but your skepticism is well-founded. As far as I can tell, no one has done this specific experiment, so we can't predict the outcome.
(If we knew the outcome, it wouldn't be science, it'd be engineering.)
Are you sure? yes | no
Its good to see your progress. I'm not really too familiar with sonic chemistry but here are my thoughts.
"The reaction would be more violent if the horn came to a smaller end (same energy, less area => greater energy density)"
Isn't this redundant with the reflector?
My other major concern is that you are trying to use the high temperatures and pressures that occur in cavitation to effect the reaction. My understanding of cavitation is that the collapse of vacuum 'bubbles' is where the high temperatures and pressures occur. If you are continually supplying a gas, it doesn't seem like there will be cavitation or collapse, just an oscillating volume of gas bubbles. I think there was a good reason that the previous experiments used dissolved gases rather than pumping them in.
Are you sure? yes | no
"In theory, the rectangular horn should generate plane waves"
Your rectangular horn is very thin, (10mm?) so your sound waves with wavelength ~50mm are not going to come out as planes, but rather horizontal cylinders that will mostly miss your reflector by passing to the side.
Are you sure? yes | no