Rocket propellants for experimental rockets are mostly made with sucrose or sugar alcohol as a fuel and containing an oxidizer like potassium nitrate and additives like Red iron oxide. Potassium nitrate is widely used as fertilizer and a food preservative, it is low cost and available in large quantities. Sugar/alkali metal nitrate based propellants are environment friendly but they have a significant lower specific impulse compared to solid rocket propellants usually used for space flights like APCP (Ammonium perchlorate composite propellant).
The combustion behavior of sugar/alkali metal nitrate based propellants can be improved by mixing the reagents on a molecular level to lower the activation energy, using an eutectic mixture of the oxidizers KNO₃ and NaNO₃ in which sorbitol will be dissolved.
But there are also drawbacks. The preparation of the propellant is very dangerous. Even an electrostatic discharge can ignite the propellant in liquid state. NaNO₃ is more hygroscopic than KNO₃. And irregular re-crystallization can lead to separation of the reagents.
Phase diagram of KNO₃ and NaNO₃:
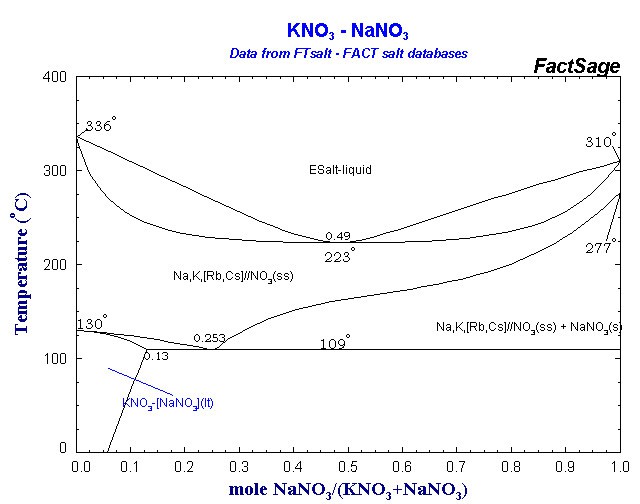
An eutectic salt mixture of for example 60% w NaNO₃ and 40% w KNO₃ has a melting point of 221°C and water-free sorbitol 110-112°C. Sorbitol has furthermore the advantage that it doesn't decompose at the melting point like sucrose.
The eutectic rocket propellant can be used in solid and in liquid state. In liquid state it has nearly a viscosity like water, so the propellant is highly cast-able in according molds and can be feed through fuel pumps.
The combustion of potassium/sodium nitrate and Sorbitol forms carbon dioxide, water, nitrogen and potassium/sodium carbonate.
I started to work on eutectic rocket propellants back in 2011 while contributing on the Sugar-Shot-To-Space program. The Russian chemist Serge77 had a similar idea by 'dissolving' Alkali metal nitrates in molten sorbitol much earlier and independent of me. According to his statement 'between 2002 and 2003 several members of the Russian Rocketry Forum lunched many rockets, using propellants with sorbitol, xylitol, mannitol and some other sugar alcohols as fuel and a dissolved alkali metal nitrates as an oxidizer, but decided then to use not fully melted propellants anymore due to safety reasons'. However, the ideas are still quite different. You can't consider a solution of sugar alcohol and an alkali metal nitrate as an eutectic system until it's proven and the solubility of an alkali metal nitrate in a sugar alcohol might be as slightly as it is for ethanol, so stoichiometric correct mixtures can't be prepared in this way and are therefore useless!
My plan to build a liquid eutectic propellant rocket engine was never realized. In 2013 an amateur rocketry group from Dubai used an eutectic composite propellant containing dissolved Lithium perchlorate in prepolymers.
Preparation
- Follow chem lab safety rules
- Put 5.4g KNO₃ and 4.6g NaNO₃ into a beaker (Borosilicate glass!)
- Mix the two substances
- Put the beaker with mixture on laboratory heating plate with temperature sensor and thermostat, insert temperature sensor into salt mixture
- Pre-set the temperature to 220ºC
- Heat till 220ºC are reached and salt mixture is liquefied (the theoretical melting point of the eutectic mixture is 218ºC)
- Add slowly 5.4g Sorbitol, stir with glass rod till the Sorbitol is molten and 'dissolved' completely in the salt bath.
- Pour the solution on a coated baking paper. Be careful, it has a viscosity nearly like water!
- Let it cool down for 10 minutes
- Ignite the mixture with a butane torch. The mixture is very difficult to ignite due to its high heat of fusion (nearly like thermite)
Combustion behavior of solid eutectic KNO₃-NaNO₃-Sorbitol propellant
Combustion behavior of liquid eutectic KNO₃-NaNO₃-Mannitol propellant
Propellant is prepared as explained above, just using Mannitol instead of Sorbitol. Mannitol has the lowest solubility in water from all sugar alcohols, therefore it is very less hygroscopic. The cured propellant has a density like ceramic. It deflagrates similar fast as KNO₃-NaNO₃-Sorbitol but without bright white flame, and a lot of white smoke is generated. In liquid state the propellant deflagrates in a split of a second though. Perform experiment only outside! Fire-proof ground and surroundings! Keep safe distance prior ignition!
Experiment set-up:

First motor test of eutectic KNO₃-NaNO₃-Mannitol propellant
182 grams of propellant in a five Bates grain arrangement. Test conducted at the FAR site, Mojave Desert California, USA on Nov 5, 2011 in support of the Sugar Shot to Space project.
 M. Bindhammer
M. Bindhammer