On my way to a better control strategy I took a closer look at the plot from yesterday. I'm interested in estimated heat capacity of the chamber and the isolation, and some sort of heat transfer coefficient between the oven's inside and the ambient air.
Here's the plot again:
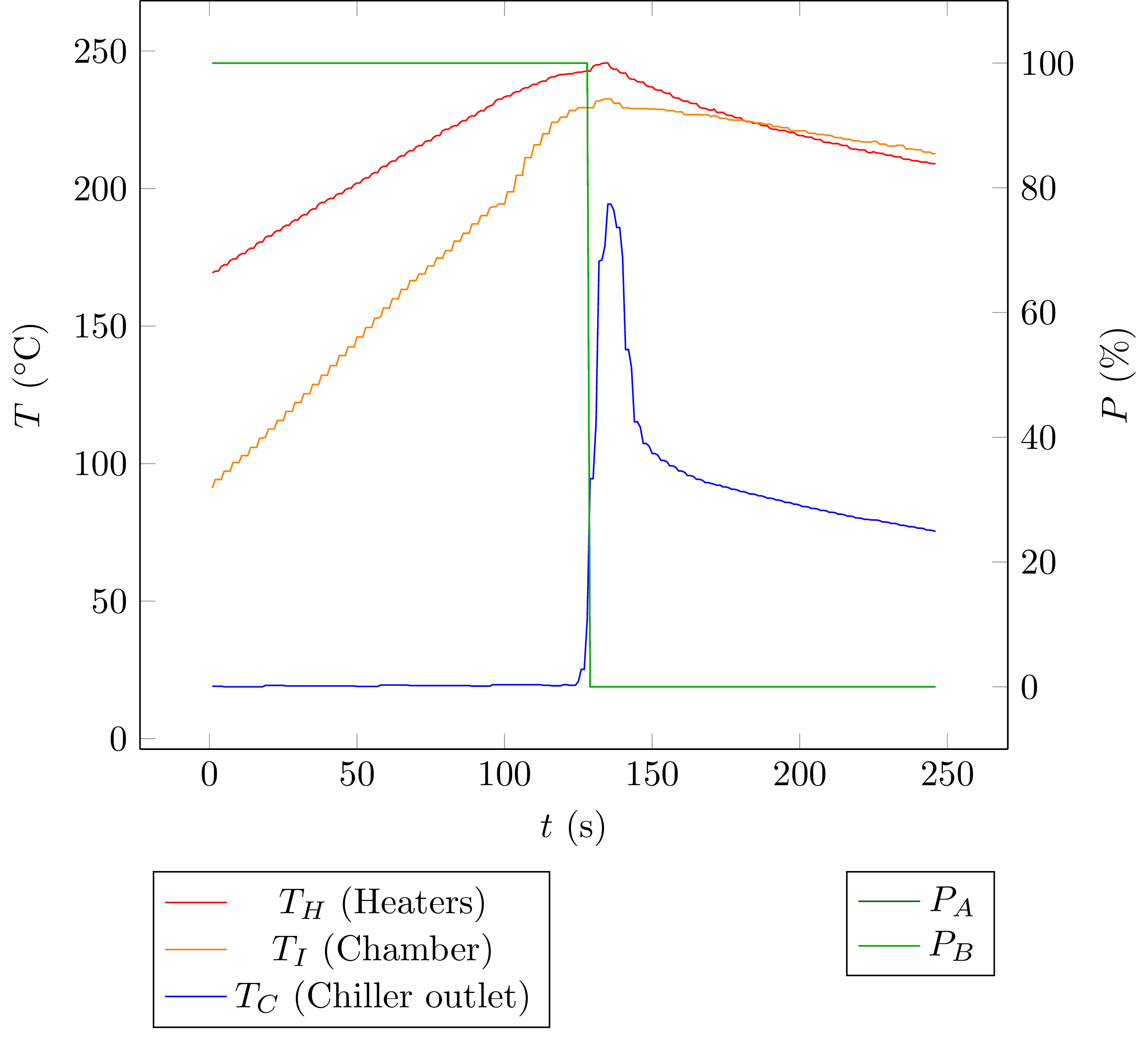
Heating up:
(index h)
The chamber's temperature rises at about 0.82 K/s, with the heaters at 400 W. At the same time, some heat will be lost to the ambient air. This rate depends on the temperature difference between heaters (let's say at t = 95 s) and ambient air, and also depends on the oven's heat capacity C:
Applying the first law of thermodynamics to the heating process gives
That's one equation with two unknowns. We need another equation to solve for them. Also note that I'm using the heaters' temperature here because the chamber is made of aluminium, and it will have a more or less uniform temperature. This is probably a better choice than using the chamber temperature, but that will come into play later.
Cooling Down
(index c)
With the heaters switched off, vapsy cools down at a rate of approximately
This is a new equation, with the same unknowns as before so we can solve for them, which gives
and thus
Calculating C is quite helpful because I can make a rough estimate for it using thermodynamic properties of aluminium and the vermiculite plates. The aluminium block weighs about 460 g with a specific heat capacity of 897 J/(kg K), the plates weigh 90 g with a specific heat capacity of about 1000 J/(kg K) and will have a temperature of about T\_I/2 because there's a steep temperature gradient inside the plates. This makes for
That's spot on for the oven's heat capacity. Let's do the same to get an estimated heat flow through the insulation (without the lid), which has an area of about 0.037 m², is 20 mm thick, and has a thermal conductivity of 0.07 W/(m K):
That leaves 80 W for the loss through the lid. If the lid was made of two touching glass plates (85 x 55 mm², thermal conductivity 0.76 W/(m K) ), each 2 mm thick, it would release
Given that the actual lid has a 4 mm air gap between the plates, the real loss will be much lower. So the estimated loss of 107 W (or 80 W for the lid only) really seems reasonable. Another factor here is that during the period that I used to sample data for the estimation, the chamber's temperature was lower than 230 °C especially during heating up. The lid is on top of the chamber and does not touch the aluminium block, so using the chamber's temperature would be better but it would also complicate things. This is good enough for now.
So what?
My next round of safety algorithms will expand on the overtemperature detection idea, but with more limits added:
- If the heaters are above 225 °C, limit power to 140 W. This will be enough to compensate for losses while still evaporating some galden.
- If the chiller exit is above 70 °C, limit power to 80 W.
- if the chiller exit is above 100 °C, cut power.
I'll implement this when I have some spare time, probably followed by a test next week.
 Christoph
Christoph
Discussions
Become a Hackaday.io Member
Create an account to leave a comment. Already have an account? Log In.