Over the course of developing the Bipolar Membrane Energy Harvester, we used many different materials. We decided to put each and every one of the materials used through a series of tests to determine its reactivity to the various solutions we’ll use in the Energy Harvester.
Our goal is to find materials that will serve our purpose without interfering with the experiment. It’s imperative that any materials used are non-reactive under the experimental conditions because with this particular design, we are looking for very small amounts of energy. Any unintended interactions could overwhelm the effect we are trying to measure.
For our purposes, we decided to test each material sample for a few different things. All the samples had their pH tested, while those potentially containing ammonia, chloride, or manganese each had those tests run as well. Any discrepancy between a control and a sample is indicative of an unfavorable reaction.
A more in-depth look at how to run the materials testing can be found in Part 2 of the instructions. Here we will discuss the materials themselves, and what sort of patterns we saw when testing them. To give a brief explanation about how we ran the tests: A small amount of each sample we wanted to test was put into four different glass vials. Each vial was filled with a different solution. Two vials of each solution were also made with no material added to act as a control. They were left for a week before measurements were taken. After the samples were put in fresh solution and allowed to sit for another week.
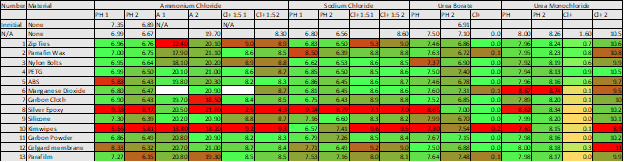
Table 1: Results from each round of testing
In table 1, you can see the measurements taken for each of the different samples tested. Samples marked in red vary significantly from what was expected. Some of the materials, like the epoxy, we expected to react. Other samples were more of a surprise and were the reason we took time to test each of the materials. Kimwipes, for example, had a major impact on the pH and chloride of most of the samples. We had assumed they were unreactive, so after learning this we made sure to adjust our methods accordingly and rinse any components that came in contact with the wipes before using.
Other materials became less reactive after the initial soaking, which means they could be used if we were careful. We also saw that the urea solutions seemed less reactive. That was one of the major reasons we ultimately chose urea borate for our first test producing a pH gradient with the bipolar membranes.
Ultimately, the diligence used when testing any and all materials allows us to be more confident in our results. The end goal of this testing was to eliminate potential interference with the effect we are trying to measure.
Discussions
Become a Hackaday.io Member
Create an account to leave a comment. Already have an account? Log In.