Olive Oil Study, using Acetone as primary solvent
The major fatty acids in olive oil, triacylglycerols are:
Oleic Acid (C18:1), a monounsaturated omega-9 fatty acid. It makes up 55 to 83% of olive oil. Linoleic Acid (C18:2), a polyunsaturated omega-6 fatty acid that makes up about 3.5 to 21% of olive oil. Palmitic Acid (C16:0), a saturated fatty acid that makes up 7.5 to 20% of olive oil.
Stearic Acid (C18:0), a saturated fatty acid that makes up 0.5 to 5% of olive oil.
Linolenic Acid (C18:3) (specifically alpha -Linolenic Acid), a polyunsaturated omega-3 fatty acid that makes up 0 to 1.5% of olive oil.
PIGMENTS AND COLOR The unique color of olive oil is due to pigments like chlorophyll, pheophytin, and carotenoids. The presence of various pigments depends on factors such as the fruit ripeness, the olive cultivar, the soil and climatic conditions, and the extraction and processing procedures.
According to Apostolos Kiritsakis, one of the premier researchers on olive oil components, fresh olive oil contains between 1 and 10 parts per million chlorophyll. This is miniscule compared to a portion of spinach. Olives are invariably crushed with some leaves still present, so some of the chlorophyll comes from that source.
Some producers have been known to deliberately allow leaves in the mill to increase the "grassiness" of the oil.
In the light, chlorophyll and pheophytin will promote formation of oxygen radicals and speed up oxidation, but in the dark chlorophyll acts as an antioxidant. In current physiological studies, chlorophyll is broken down in the body and has no effect as an oxidant or antioxidant.
Molecular weight [282.47 g/mol] oleic acid + (Linoleic Acid, Palmitic Acid and Stearic Acid) Chemical Formula Extra Virgin Olive Oil: C18H34O2 Density: 0.895 g/ml Soluble in Ethanol
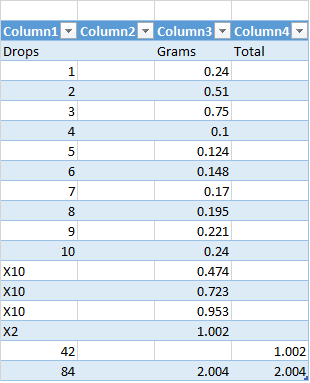
I created this weight table for easier calculation inputs in Excel when determining which sample concentrations I want to work with. *note, sample weights are relative to volume of drop size exiting glass eye dropper.
Using acetone as my primary solvent for this study was appropriate because it has both polar and non-polar properties, which are ideal in the separation of the constituent compounds of chlorophyll A,B and B-carotene (which is non-polar, due to it begin a hydrocarbon.)
As you can see in the table, 42 drops of olive oil equals 1000mg and 84 drops equals 2000mg. Which also equate to 1ml and 2ml in volume. Each cuvette has a total capacity of 3ml, but I only used a mixture of 90% acetone and 10% distilled water as per the literature's experimental procedures. So the sample with 2000mg had a total solvent volume of 0.5ml and the 1000mg sample had a total solvent volume of 1.5ml.
Each sample was scanned for absorption using forward facing geometry for maximum gain. All dark counts have been removed.
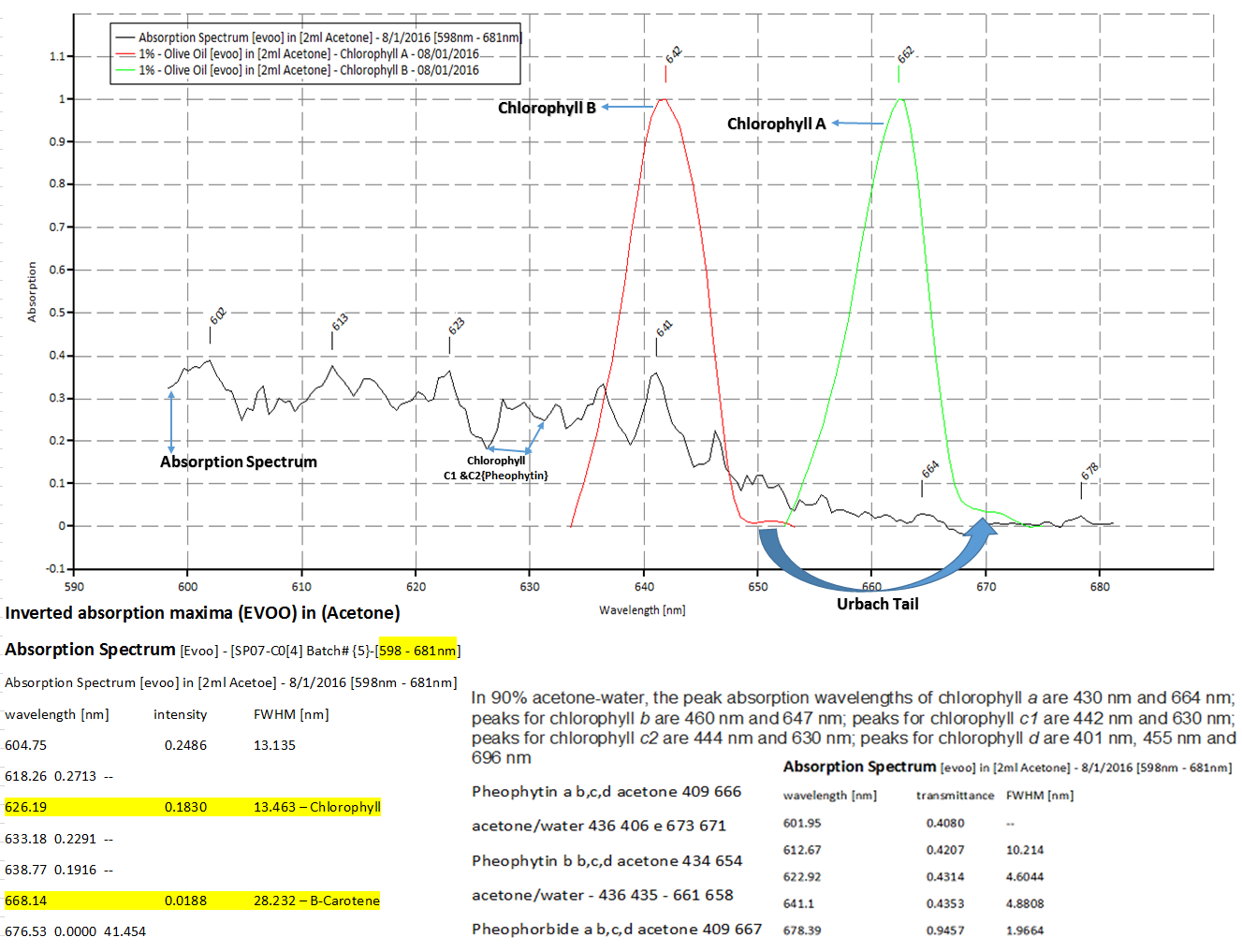
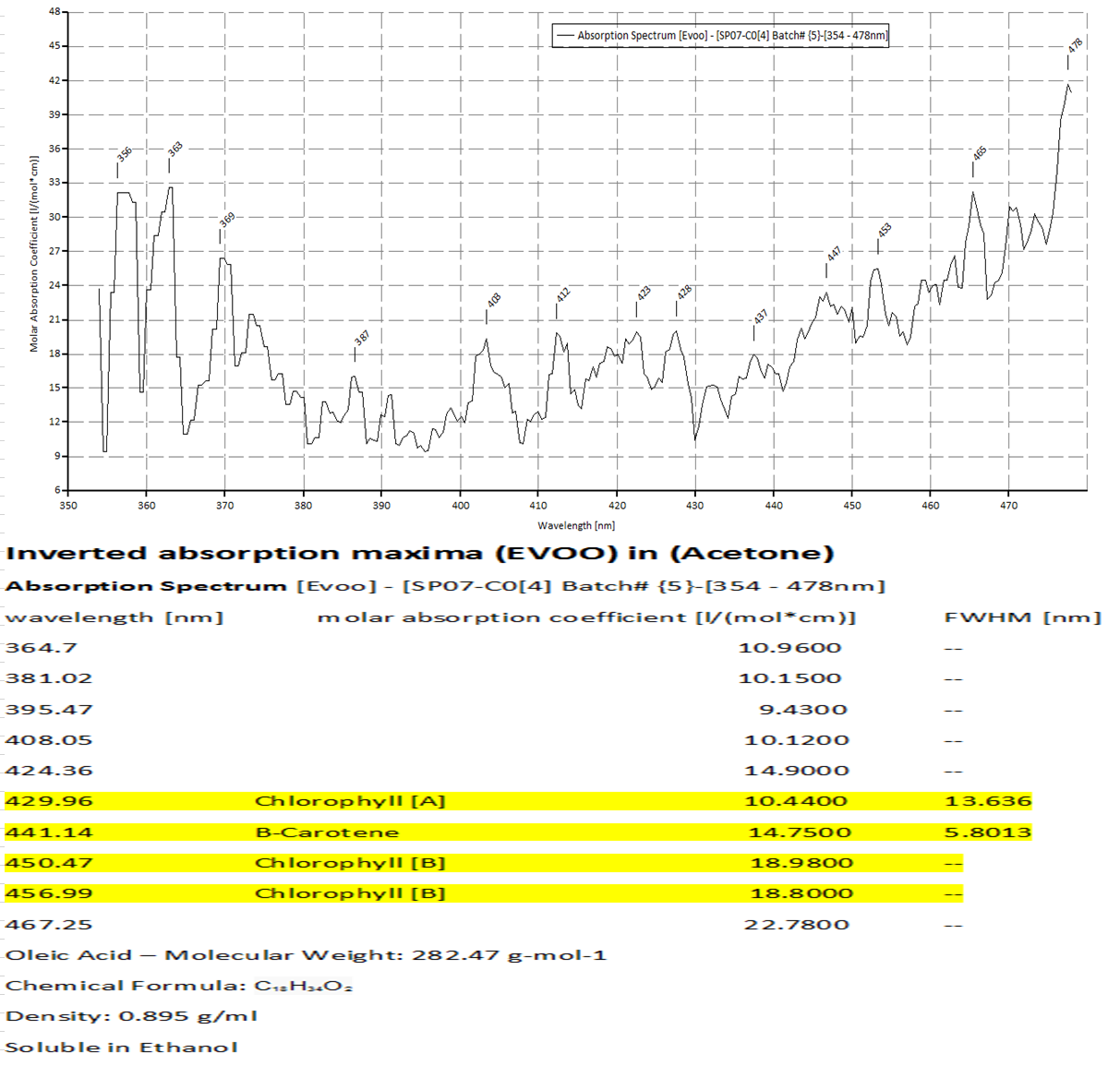
The above chart corresponds to the next plot (which is the emission data for chlorophyll A and B.) You see in the highlighted area, that at 429.96 is the absorption band for chlorophyll A, and at 450.47 is for chlorophyll B. in the plots below are emissions for chlorophyll A at 662nm and chlorophyll B at 642nm.

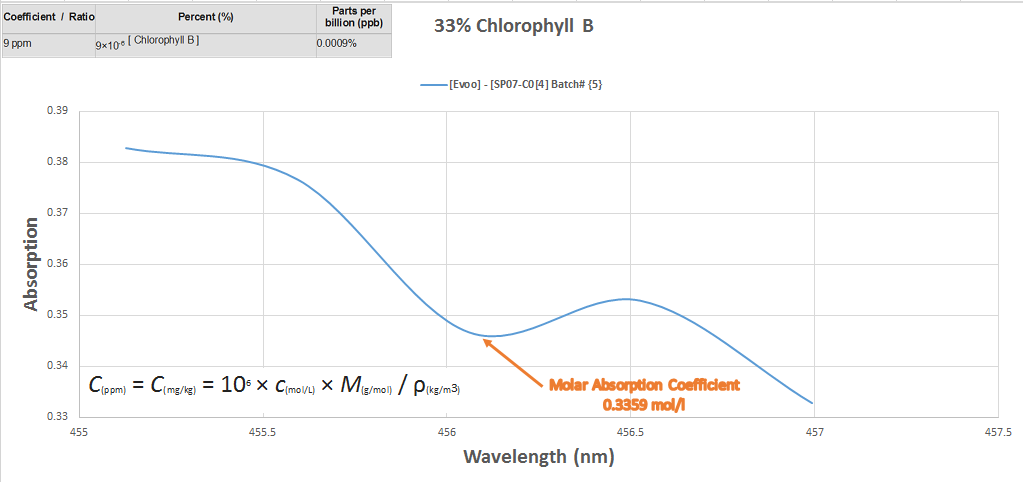
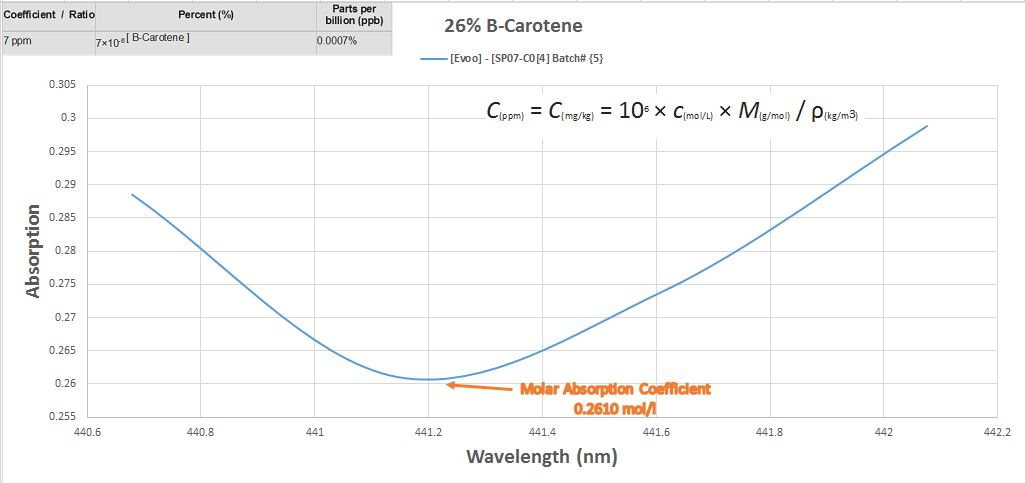 The next chart is the actual chemical concentration percentages contained within the oil itself, explained
The next chart is the actual chemical concentration percentages contained within the oil itself, explained
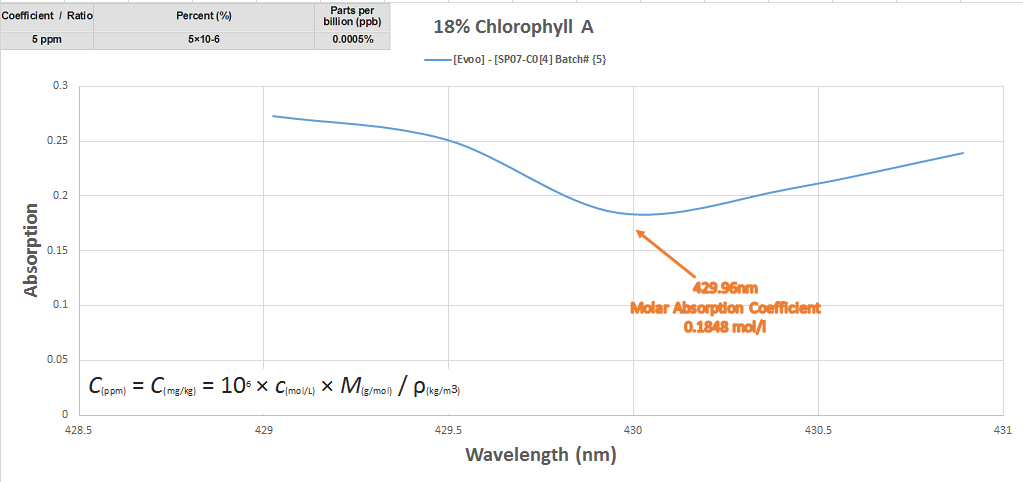
 The last chart explains the relationship between the intensity of absorption as it relates to the concentration at transmittance of the substance to parts per million converted per milliliters.
The last chart explains the relationship between the intensity of absorption as it relates to the concentration at transmittance of the substance to parts per million converted per milliliters.
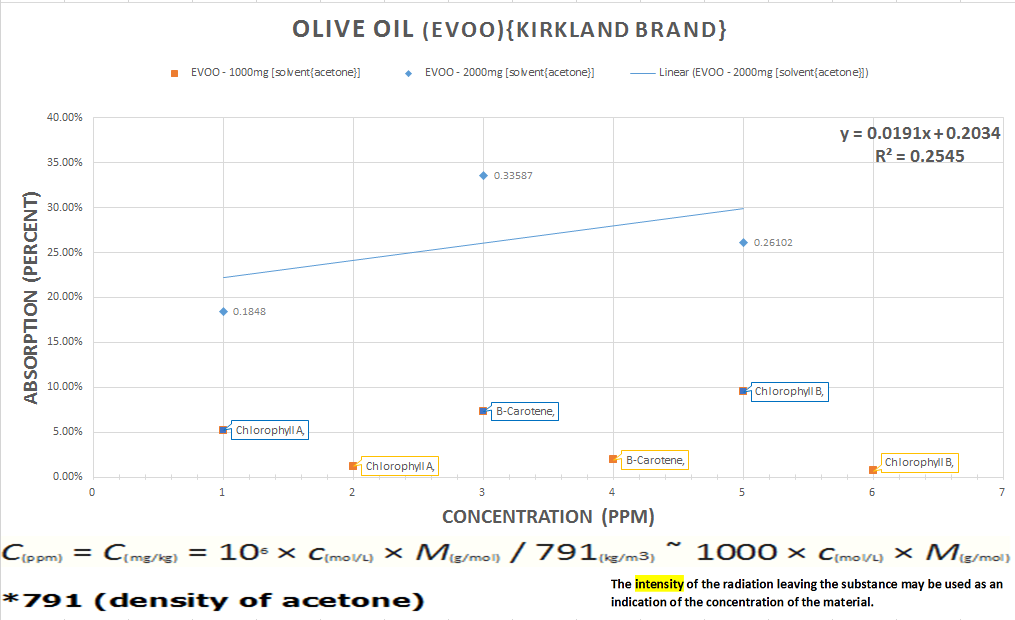 in conclusion, this is further validation on the viability and reliability for repeatable results, to a comfortable degree (in my opinion) of confidence for intermediate chemical analysis of a variety of compounds using this type of spectrometer.
in conclusion, this is further validation on the viability and reliability for repeatable results, to a comfortable degree (in my opinion) of confidence for intermediate chemical analysis of a variety of compounds using this type of spectrometer.
http://en.wikipedia.org/wiki/Oleic_acid
http://cdn.intechopen.com/pdfs/27027/InTech-Analysis_of_olive_oils_by_fluorescence_spectroscopy_methods_and_applications.pdf
http://www.chemicalbook.com/ChemicalProductProperty_EN_CB3120475.html
http://en.wikipedia.org/wiki/Pheophytin
http://en.wikipedia.org/wiki/Chlorophyll
http://www.slideshare.net/nikhilbinoy/uv-and-visible-absorption-spectroscopy
 David H Haffner Sr
David H Haffner Sr
Discussions
Become a Hackaday.io Member
Create an account to leave a comment. Already have an account? Log In.