Solvay, the manufacturer of Galden LS 230, publishes the thermophysical properties of that substance at 1 bar and/or 25 °C. That's not even close to the temperature it's actually put to good use at - that would be 230 °C or even more during vaporization.
Here's what we get at 1 bar / 25 °C:
- Normal boiling point: 230 °C
- Density: 1820 kg/m³
- Kinematic viscosity: 4.4 cSt = 4.4 x 10⁻6 m²/s
- vapor pressure: 3.4 torr = 453.3 Pa
- specific heat: 973 J/kgK
- heat of vaporization at boiling point: 63 kJ/kg
- thermal conductivity: 0.07 W/mK
- coefficient of expansion: 0.0011 1/K
- surface tension: 20 dyne/cm = 0.02 N/m
- average molecular weight: 1020 a.m.u, so molar mass: 1.02 kg/mol
- some more I don't expect to need:
- dieletric strength
- dielectric constant
- volume resistivity
Vapor density
I was able to find a plot of vapor density over temperature for Galden HS 260 (this is a different type than I'll be using) in a solvay presentation somewhere in the depths of the internet:
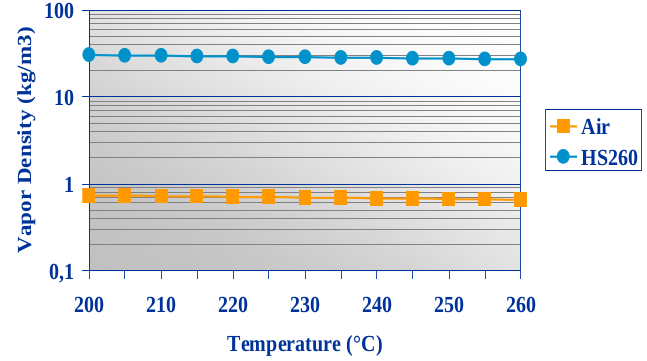
It's a crappy plot, but helped my anyway.
For this Galden type we can ballpark the vapor density at 260 °C (assuming 1 bar here) to be somwhere between 24 and 30 kg/m³. Galden HS260 has an average molecular weight of 1210 a.m.u, or a molar mass of 1.21 kg/m³. Applying the ideal gas law
yields 27.3 kg/m³ at 260 °C. That's within the range I identified in that plot up there, so I guess it's ok to just apply the ideal gas law to Galden to figure out the vapor density (this is not necessarily a given). For the LS 230 grade, the result is 24.4 kg/³ at 230 °C. For comparision, air has a density of about 0.7 kg/m³ at that temperature.
The higher density of galden vapor has the effect that galden vapor released into the space above boiling galden will not violently escape like water vapor in your kitchen, but will tend to stay close to the surface. Some of it will mix with air and the air's galden content will decrease as we move upwards from the surface.
To conclude: Galden vapor is heavy, and it's probably ok to use the ideal gas law for it.
Specific heat, heat of vaporization and thermal conductivity
Specific heat will probably rise with temperature, as it does for many common substances. I'll assume it to be more or less constant for now.
Heat of vaporization is only needed at atmoshperic pressure, or pretty close to that. No need to get into that in more detail.
Thermal conductivity...my gut feeling is that it won't change a lot between 25 and 230 °C. I might be wrong, though.
Other properties will be addressed in the next log.
 Christoph
Christoph
Discussions
Become a Hackaday.io Member
Create an account to leave a comment. Already have an account? Log In.