At the heart of the project is finding an alternative for the PEM. One of the biggest issues that I have run into is that there very little information out there about exactly how PEMs are contructed. There is a lot of information available regarding some of the materials used and the chemistry of the process, but I have yet to find a good cut away image of a commercially manufactured cell or PEM.
FYI.... If you contact hydrogen fuel cell manufactures asking for more information... They don't respond.. Or they do, but it's a blanket statement that it's a trade secret, and ask you not to contact them again... :/
So what to do.... what to do.... Well as Dr. Whitehall so eloquently put it "Discovery requires experimentation.... Hail Hydra!" Sorry had to get the mad scientist bit out of the way.
Through my research, I have found that a PEM cell seems to be comprised of an anode and a cathode with an electrolytic membrane sandwich between the two along with a catalytic layer on both the anode and cathode sides.
Here's a few generalized diagram of what the PEM cell should look like:
---------- PEM Fuel Cell----------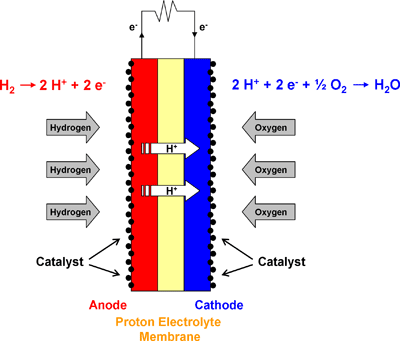
The reaction needed for a PEM fuel cell to function is a redox reaction. This means that we need to take the single electron from the hydrogen atom through an oxidation state and deliver that electron to the other side of the cell where hydrogen will meet up with oxygen and the spare electron to create water in a reduction state.
Here's a link to the Wikipedia page explaining redox reactions
---------- Hydrogen Atom---------
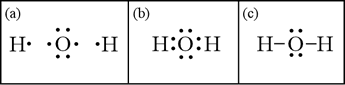
---------- Bonding of Hydrogen To Water----------
---------- Diagram of Reaction In Fuel Cell----------
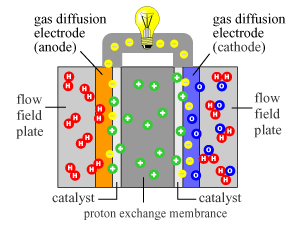
Catalysts:
With that last bit out of the way, it's time we discuss catalysts. There are three major metal that are unique in their ability to catalyze reactions between hydrogen and oxygen. Those are platinum (Pt), palladium (Pd), and rhodium (Rd). Unfortunately these metals are both rare and hard to work with. But fortunately there are several possible sources where these metals exist in a form that is more or less easier to work with. The most common sources that I could find these metals are in catalytic converters and hard drives.
- I have thought about using slightly roughed hard drive platters stacked as both a catalyst and for the anode and cathode. I do not know if this will work as I have a rudimentary understanding of chemistry, and I am unaware of Pt, Pd, and Rd compounds present on hard drive platters. As I am unsure of this, I am also unsure of whether it will actually work as a catalyst, but I'll be sure to test.
- I have also thought about cutting sections out of a catalytic converter from an internal combustion engine, as they are specifically manufactured to catalyze hydrogen and oxygen compounds. However, I has been told that the support material in some catalytic converters may contain asbestos. Further research is needed, bc I don't like cancer, and would feel pretty terrible about encouraging others to potentially contaminate themselves.
- Another approach would be to harvest the catalyst metals and convert them into a metallic salt as suggested in the comments by Jehu:
" ...The way I'd get the Pt out is to snap the platters, submerse in NaOH to react the Al, wash and roast the Pt foil and then react with Aqua Reiga to make a chloro platinate salt."
Membrane:
Now we have come to what I believe will be the most challenging part of the build; The PEM. The reason I feel like this is the most changeling part is that finding a material that will only allow a single proton (.1 µm) to pass through and/or acidic enough to assist with knocking the electron off of the hydrogen atom, while allowing the hydrogen proton to pass through the membrane to the cathode side of the fuel cell. This also means that the both the anode and cathode would need to separated via a barrier from the acid.
- One of the primary materials used as a PEM is a chemical cousin to Teflon (PTFE), known as Nafion. It's a pretty amazing material that conducts protons but not electrons. (Check out the Wikipedia page for Nafion) For the sake of this project Nafion is a dead end in the project due to it's extremely high cost. The same applies for any commercially available that i can find. (Link to a pricing list of PEMs at Sigma-Alrich
- I've also been kicking around the idea of doping Tyvek (Official website here) with catalyst and ceramic and then stacking layers tightly together, and then sandwiching a common mild acid (preferably a solid) between the layers
- I have read bits and pieces about using silica as a PEM, but have been unable to find any substantial information.
Thanks for stopping by! If you have any suggestions or questions, please feel free to comment and share. If you have a chemistry background and see anything that I have screwed up, please feel free to correct me.
 Chali Baicunn
Chali Baicunn
Discussions
Become a Hackaday.io Member
Create an account to leave a comment. Already have an account? Log In.