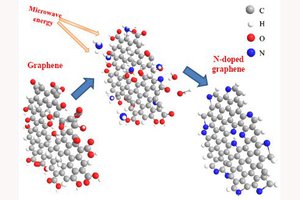
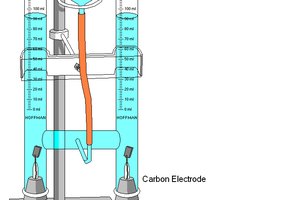
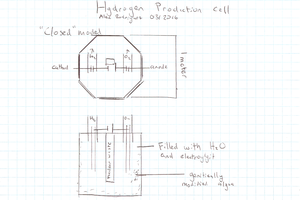
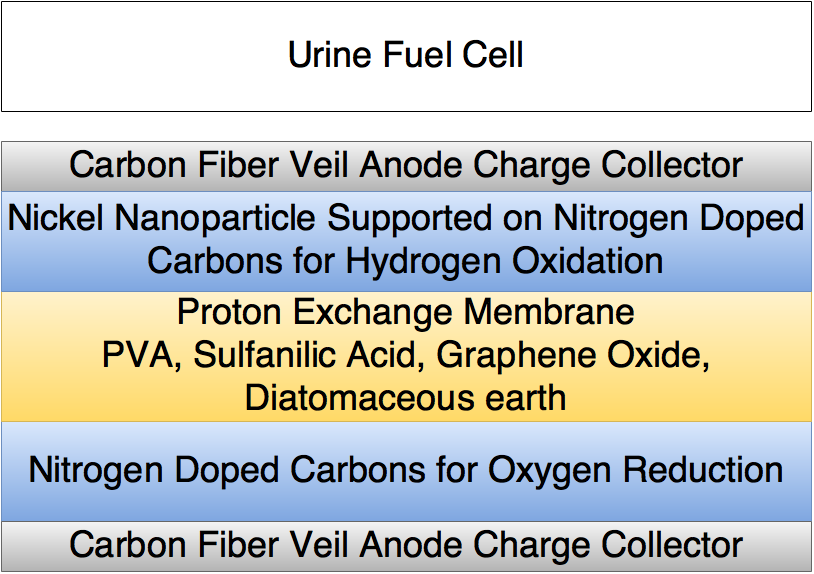
Nitrogen doped carbons make great cathode materials as they readily catalyze oxygen reduction reactions combining 2 hydrogen protons 2 electrons and an oxygen atom to form water.
However nickel nano-particles are needed to catalyze hydrogen oxidation reactions at the anode. These must be supported on nitrogen doped carbons for maximum effeciency so when reducing nitrogen doped carbons I will also be reducing nickel acetate. With l-acorbic acid as the reduction agent the nickel nano-particles can be deposited on the nitrogen doped carbons.
The low temperature PEM(proton exchange membrane) will be made using sulfanilic acid doped poly vinyl alcohol containing graphene oxide and amorphous diatomaceous earth.
Hydrogen will be evolved by bacterial reduction of urea into ammonia.
This documentation describes Open Hardware and is licensed under the CERN OHL v. 1.2. You may redistribute and modify this documentation under the terms of the CERN OHL v.1.2. (http://ohwr.org/cernohl). This documentation is distributed WITHOUT ANY EXPRESS OR IMPLIED WARRANTY, INCLUDING OF MERCHANTABILITY, SATISFACTORY QUALITY AND FITNESS FOR A PARTICULAR PURPOSE. Please see the CERN OHL v.1.2 for applicable conditions
 MECHANICUS
MECHANICUS