A hazard analysis of the OpenFluidWarmer sous vide cooker design was performed using a reduction as-far-as-possible (AFAP) risk management approach (the latest revision of the hazard analysis is "OpenFluidWarmer_HazardAnalysis" in the project files). The goal of the hazard analysis is to identify the harms and develop the mitigation controls for each significant hazardous condition that can occur during device operation. EN ISO 14971, an ISO standard detailing the application of risk management to medical devices, was used as a reference to perform the analysis.
The OpenFluidWarmer hazard analysis identified six hazards (all with multiple potential causes) and four resulting harms. Many risk controls were identified to further reduce the probability of a hazard condition occurring, but none were identified that would reduce the severity of the harm once a hazard condition does occur.
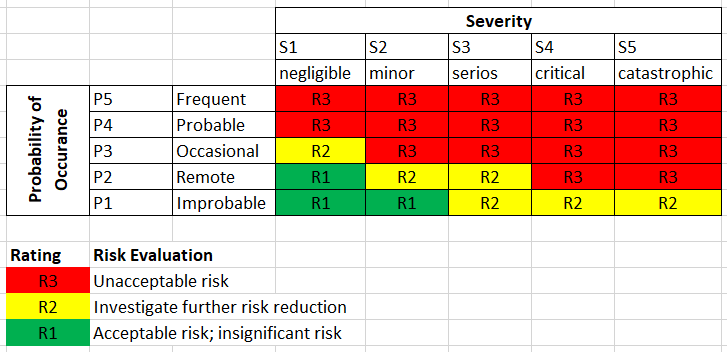
When following the AFAP approach, risk is to be reduced to the R1 rating (per image above) regardless of economic practicality. In cases where it is not possible to reduce risk to a R1 rating, a risk-benefit analysis is to be performed. The risk-benefit analysis must provide evidence (with no consideration given to economic practicality) that the medical benefits of using the device outweigh the residual risks.
The reduction as-far-as-possible risk management approach provides an exciting opportunity to explore the feasibility of electromechanical open-source medical devices like the OpenFluidWarmer. With the number of risk reduction controls required, adherence to AFAP risk reduction presents the most difficult cost challenges for the OpenFluidWarmer design. On the other hand, if it is possible to follow the AFAP risk reduction approach and achieve a low-cost, easy-to-manufacture, component-flexible design, that has huge implications beyond the development of just the OpenFluidWarmer. It means that it may be possible to develop other safe and economically practical open-source medical device designs; some that may even provide more medical benefit to the patient than IV fluid warmers.
 John Opsahl
John Opsahl
Discussions
Become a Hackaday.io Member
Create an account to leave a comment. Already have an account? Log In.