Summary:
.) Call for collaborators
.) Didn't make the cut
.) New board design should be better
.) New reaction vessel should be... interesting
.) Entropy and the Haber process
Call for collaborators
If you'd like to join the project, ask to join my GitHub groups: Sonicators, for people who want to develop and build the system, and/or AmineGroup, for people who want to design and conduct experiments (of any type, not just ammonia ones).
Or contact me through the Hackaday.io.
Didn't make the cut
The project didn't make it into the next round of the contest. This is a little disappointing, but also a relief - keeping up with the contest requirements takes a fair amount of time and constant stress.
I'm now free to proceed at a more leisurely pace.
The intent of the project was to build a system capable of exploring ultrasonic chemistry, and I've done that. I now have 3 systems capable of ultrasonic processing, and can easily make more if needed.
A big thanks to everyone following the project and who gave feedback and support with skulls and such. I really appreciate the interest, and I hope you enjoyed the build log dialogs.
Since the project focus will now be on chemistry (and not electronics), I'll be adding build logs at a slower pace, and probably using the GitHub wiki instead of the Hackaday system. I find the Hackaday system very difficult to use, and the results aren't really easy for others to view.
New board design should be better
I had frozen the electronics for purposes of the contest: it did all the things I needed it to do, there was no compelling reason to change it.
Since then, I've come up with some new features that shouldn't be too hard to implement - some things that make the board easier to build, some convenience features, and some for a more robust project.
1) The board as designed allows the user to sweep the frequency and determine the resonance point of the transducer/horn using a separately plugged input. Having this sweep without plugging/unplugging the transducer would be a convenient feature to have. I think the appropriate choice would be to use a relay on the transducer output that automatically switches the transducer between the low-voltage sweep and the high-voltage power output as needed.
2) The current design requires a center-tapped SMPS transformer. These are available but uncommon (about 10% of all ATX supplies I've looked at), and being able to use a single-winding device would allow the user to use any transformer of suitable power. Using a pre-made class-d amplifier might accomplish this. A pre-built class-d smplifier board on eBay for around $30 (more or less, depending on power) might suffice, and would reduce the amount of soldering the user has to do.
3) A higher voltage power supply makes everything easier: the trace widths for 10 amps (12 volts) is around half an inch, which are nigh impossible to route on a board. Printer or laptop power supplies in the 20-40 volt range are available for thin money on eBay ($10). This would allow the circuit board to use thinner traces. Also, the higher input voltage means a higher transducer output voltage, so the system could push more power through an off-resonance transducer.
All told, the driver board will go through another rev of design, and V2.0 will be easier to build, easier to use, and more robust.
New hardware has been ordered - waiting for delivery.
New reaction chamber
Now that I've got an ultrasonic system, it's time to do some experiments!
I've discovered that holding a bubble underwater is a hard problem, partly due to physics and partly due to currents in the reaction chamber.
To get around the latter, I've been mulling over some new reaction chamber designs.
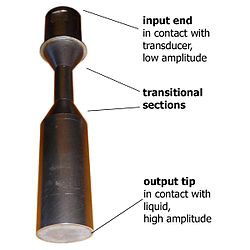
A type of horn called a "barbell" is sometimes used for ultrasonic processing. It's tuned to the resonant frequency of the transducer, and the weighted ends vibrate back and forth, as expected.
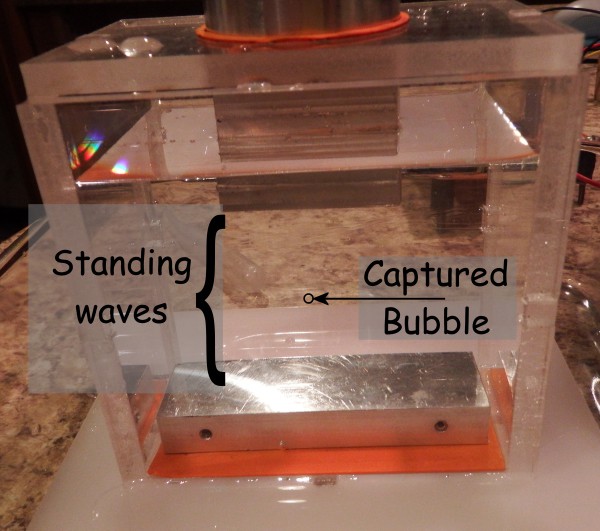
Additionally, most ultrasonic experimental setups use a "tuned chamber", which makes use of standing waves in the conducting media (usually water).
So...
I'm wondering if water itself can be used as an ultrasonic resonant horn.
Water has a bulk modulus of around 2 gPa (about 20,000 atm), which should be more than adequate for a resonant horn at the power levels we're dealing with.
A polycarbonate tube in the shape of an hourglass should concentrate the ultrasonic energy according to Laplace's law. Anything in the restricted section (the "waist") of the tube should experience an enormous energy density.
Furthermore, an inlet at the bottom and an outlet at the top should force induced bubbles to pass through the narrower section.
Further furthermore, tuning the system is simply adjusting the height of the water column in the hourglass. A tuned horn with an O-ring seal and appropriate height adjustment should be able to easily allow the user to create standing waves within the chamber.
It turns out this is an actual thing, it's called a "flow through reactor", and made by a company sonomechanics.com. (I'd show a picture, but their image is copy-protected.)
So I've got some polycarbonate tubes on order, and will be playing around with new and improved reaction chambers in the near future.
Entropy and the Haber process
Converting Nitrogen is seemingly easy: the reaction is exothermic, so we only need to heat the source gases and Ammonia should form. The excess energy can heat more gas, so the conversion should be self-sustaining, like a flame. Ignite the fuel, give it a continuous supply, and collect the combustion products.
Easy peasy.
Unfortunately, it doesn't work that way. Whether a reaction will occur depends not the release of heat, but on the release of entropy.
The Haber reaction is exothermic, and this release of heat increases the entropy of the universe. At the same time, 4 atoms of source become 1 atom of product, and this *decreases* the entropy of the universe. There's more ways that 4 atoms can be arranged in a box than there is to arrange 1 atom.
We can calculate the amount of entropy lost (or gained) in the reaction by subtracting the entropy of Hydrogen and Nitrogen from the entropy of Ammonia:
Entropy from the release of heat is just the energy divided by the temperature. Since it's inversely proportional to temperature, doubling the [absolute] temperature will halve the increase.
At room temperature, the entropy increase from the release of heat is greater than the entropy decrease from the reduction in states, so the reaction is favored. At higher temperatures, the entropy increase from "release of heat" is smaller than the entropy decrease from "change of states", the total change of entropy is negative, and the reaction is no longer favored.
To ignite the reaction you first need to break N2 molecules into
individual N atoms, and this requires a great deal of initial energy
which is regained in subsequent steps. The molecules in normal air have a bell-curve
spread of energies, and very few have enough energy to react: at room temperature only a handful
of molecules will be converted each second.
Raising the temperature increases the probability that molecules will have enough energy to react, but then the entropy from released heat no longer outweighs the entropy decrease from the reaction, so the reaction is not favored.
It's catch-22: At room temperature the reaction is favored but cannot start. At high temperatures, the reaction can start but isn't favored.
Increasing the pressure of the reactants will tend to favor the products so you can use this to offset the deficit in entropy.
The Bosch-Haber process tries to find a "sweet spot" by increasing the temperature to get a reasonable number of N2 molecules to break apart, and high pressure to make the process favor the products.
At 200 ATM and 400 degrees, the yield is 15%.
Reaction
vessels for this pressure and temperature are expensive, and the
process requires multiple cycles of compression, decompression, removal
of ammonia, and recompression. This takes a *lot* of energy and uses
*very* expensive compressors which wear out over time and have to be
replaced.
This is why the Haber process is energy intensive.
 Peter Walsh
Peter Walsh
Discussions
Become a Hackaday.io Member
Create an account to leave a comment. Already have an account? Log In.